Figure 3.
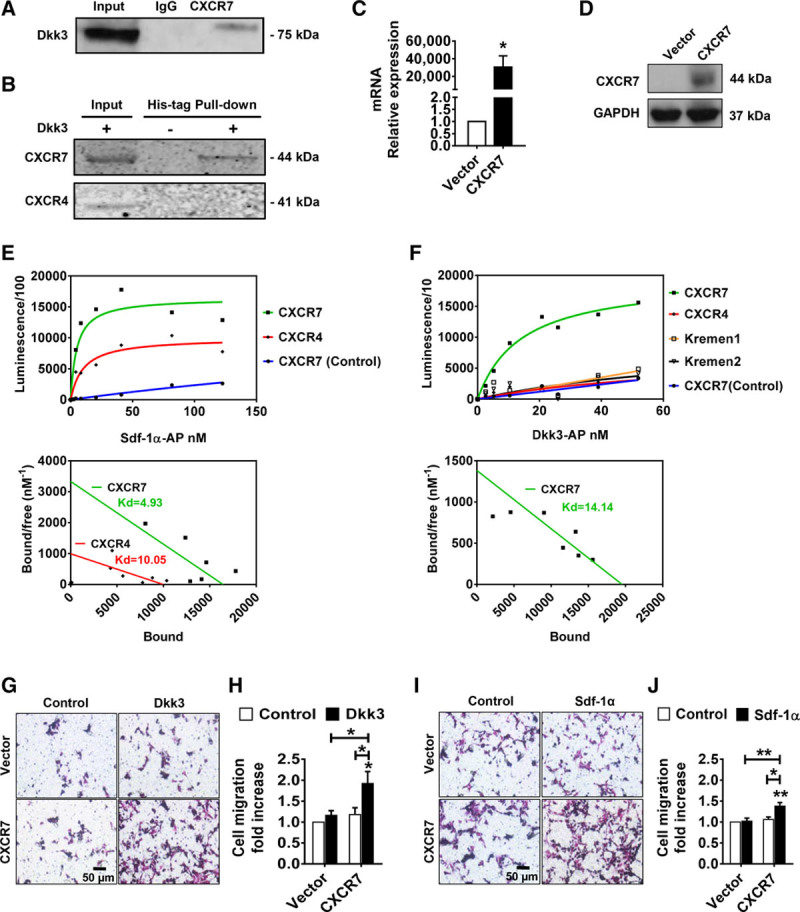
CXCR7 is a high-affinity binding receptor of Dkk3 (dickkopf-3). A, Coimmunoprecipitation of Dkk3 with CXCR7. Dkk3 coimmunoprecipitates with CXCR7 from Sca-1 (stem cell antigen-1)+ cells. B, Immunoblotting of the receptors pulled down from Dkk3-His tagged, using Nickel magnetic beads. Sca-1+ cells were treated with Dkk3-His tagged. CXCR7, but not CXCR4, is pulled down with Dkk3-His. C, Quantitative real-time quantitative polymerase chain reaction analysis of CXCR7 mRNA expression in HEK 293T cells transfected with CXCR7 expression plasmid. Expression levels were normalized against GAPDH (n=4; 2-tailed unpaired Student t test). D, Western blot analysis of CXCR7 overexpression in HEK 293T cells transfected with CXCR7 expression plasmid. E and F, Representative binding curves and respective Scatchard analysis of Sdf-1α (stromal cell-derived factor 1α)–alkaline phosphatase (AP) binding to CXCR4 or CXCR7 overexpressed in HEK 293T cells and of Dkk3-AP binding to CXCR7, CXCR4, Kremen1, or Kremen2 overexpressed in HEK 293T cells, respectively. The dissociation constants are represented for each receptor (n=3). Dkk3-AP does not bind to CXCR4, Kremen1, and Kremen2, but it does bind with high affinity to CXCR7, as represented by the characteristic hyperbolic binding curve. CXCR7 is also a high-affinity binding receptor of Sdf-1α, alongside its cognate receptor CXCR4. AP alone does not bind to CXCR7, as depicted in blue. G and I, Representative images of transwell migration assay of HEK 293T cells overexpressing CXCR7 in response to Dkk3 and Sdf-1α stimulation, respectively. H and J, Quantitative analysis of the transwell migration assays. Dkk3 induces migration of CXCR7-overexpressing HEK 293T cells, analogously to Sdf-1α. (n=5; 2-way ANOVA followed by Bonferroni post hoc test). The data are expressed as the mean±SEM of 3 to 5 independent experiments. *P<0.05, **P<0.01, compared with control group.
