Abstract
Objective
In this retrospective study, chemotherapy induced amenorrhea in patients with early stage breast cancer and its effects on survival were investigated.
Materials and Methods
Two hundred fifty-two patients received adjuvant chemotherapy without ovarian suppression treatment (OST) from 600 premenopausal patients were included in the study. Patients were divided into two groups; with amenorrhea and without, and compared with clinicopathologic features and survival. SPSS version 17 was used.
Results
Chemotherapy-induced amenorrhea (CIA) was observed in 145 (57.5%) of 252 patients who received no OST during follow-up. The 5-year OS rate of patients with CIA was significantly higher than patients without CIA (p= 0.042, 95.9% vs. 89.7% vs. 158.88 vs. 135.33 months, respectively). In the subgroup analysis, the OS in patients with hormone receptor (+) was significantly higher than in those receptor (−) in patients with CIA (p=0.011, 97.5% vs. 90.9% vs. 162.13 vs. 126.16 months, respectively). The OS was significantly longer in the luminal A molecular subtype than in those with luminal B molecular subtype, in patients with CIA, but the difference was not significant in patients without CIA (p=0.027 vs. p=0.074, respectively).
Conclusion
As a conclusion; survival advantage of the chemotherapy induced amenorrhea more pronounced with hormone receptor positivity, lymph node involvement, and advanced disease over patients who do not develop amenorrhea. This advantage of amenorrhea development further prolongs survival compared with luminal B in the luminal A molecular subtype.
Keywords: Chemotherapy-induced amenorrhea, molecular subtypes, breast cancer, survival
Introduction
Twenty-five percent of the breast cancer (BC) population are under the age of 50 years and premenopausal in developed countries, whereas 50% of patients with BC are premenopausal (and/or <50 years of age) in developing countries (1, 2). It has been suggested that adjuvant chemotherapy is more effective in premenopausal patients with breast cancer than postmenopausal patients (3, 4). In addition, many papers on amenorrhea as a result of chemotherapy suggested it could prolong survival. Despite chemotherapy-induced amenorrhea (CIA) being a good prognostic factor for premenopausal patients with breast cancer for 3 decades, there are few prospective trials (5–7). The percentages of CIA differ depending on patient’s age and chemotherapy regimen (8, 9). As a result of chemotherapy, the natural aging process of the ovaries accelerates, because steroid-secreting cells (granulose and theca cells) and some of the primordial follicles are damaged and follicular failure develops (10). It is considered that; survival gain is more significant because of amenorrhea with ovarian suppression besides the cytotoxic effect of chemotherapy. The survival elongation of premenopausal patients with breast cancer is more prominent with ovarian suppression treatment (OST) compared with no OST (11). Surgical oophorectomy and radiotherapy have frequently been used for ovarian suppression in the past (12). It has been revealed that OST (GNRH analogy), surgical oophorectomy and radiotherapy have similar effects on amenorrhea (13, 14). In addition, OST and CMF chemotherapy have the same survival effects on premenopausal patients with hormonal receptor-positive breast cancer (15, 16). As such, GNRH analogues are currently mostly used for amenorrhea in premenopausal patients with breast cancer.
It is considered that, the survival effect of CIA depends on the degree of hormonal receptor positivity, patient’s age, and clinicopathologic characteristics. It is necessary to plan treatments according to molecular subtypes, because different molecular subtypes have different survival outcomes and clinicopathologic characteristics. It has been shown that local-recurrence-free survival (LRFS) of luminal A subtypes is longer than in those with luminal B subtypes when we treat with OST (17). Although survival gain is only for patients with hormonal receptor-positive breast cancer, it is not known whether this effect is more prominent in luminal A or B.
We aimed to analyze the survival effects of CIA according to different molecular subtypes of premenopausal women with early-stage breast cancer regarding development of amenorrhea due to adjuvant chemotherapy treatment.
Materials and Methods
Patients’ data were identified retrospectively from the archives of the study group, between December 2000 and December 2013. Patients in this study provided informed consent for their information to be stored in the hospital database and used for research. Inclusion criteria were receiving chemotherapy and being premenopausal at the time of diagnosis, with clinically early-stage BC. Patients were excluded from study if they received neoadjuvant chemotherapy, had bilateral breast cancer or had less than two years of follow-up and had been treated with OST. The patients received no OST, were analyzed regarding the development of CIA. Analyses were performed according to pathologic characteristics such as pathologic stage, lymphovascular invasion (LVI), histologic grade (modified Scarff-Bloom-Richardson grading), presence of in situ carcinoma, multicentricity/multifocality (MC/MF), Ki 67%, immune-histochemical receptor status (estrogen receptor (ER), progesterone receptor (PR)), and human epidermal growth factor (HER2). Besides, the patients’ demographic features, adjuvant treatments, and molecular subtypes were recorded. Molecular subtypes were identified according to St Gallen 2013(18): [Luminal A: ER (+) and PR (≥20%), Ki 67 <20%, Luminal B: ER (+), PR (<20%) and/or Ki 67 ≥20% and/or Her 2 (+), Her 2 over: ER(−), PR(−) and Her2 (+), Triple-negative: ER(−), PR(−), Her 2(−)].In this study, amenorrhea lasting at least six months within two years after the start of chemotherapy was accepted as CIA. Premenopausal status is defined having regular menstruation at least for one year.
Statistical Analysis
Variables were given as means±standard deviations. Median, minimum-maximum were calculated unless otherwise specified. The distribution of variables was analyzed with Kolmogorov Smirnov Test and the quantitative analysis of variables were done with chi-square test. Overall survival (OS) was calculated from the date of surgery to the date of breast cancer- specific death or the last follow-up. LRFS and distant metastasis free survival (DMFS) was calculated from the date of surgery to the date of local recurrence or distant metastasis respectively. Also, disease free survival (DFS) was calculated from surgery date to both local recurrence and distant metastasis. Survival analysis was estimated using the Kaplan-Meier method. Univariate Cox regression models were used to evaluate the effect of each specific parameter. Multivariate Cox regression models were performed to specify the major significant predictors for death occurrence. The statistical results were considered significant at a p value <0.05. All statistical tests were performed using SPSS software version 17 (IBM Corporation, New York, USA).
Results
The median age of the patients was 39 years (range, 24–54 years). Medical OST treatment was performed in 137 (35%) of 389 patients. Chemotherapy-induced amenorrhea (CIA) developed in 145 (57.5%) of 252 patients who received only chemotherapy without OST. The molecular subgroups of patients without OST as follows: Luminal A (n=80; 32%), luminal B (n=99; 39%), Her 2 (+) (n=25; 10%), triple (−) (n=48; 19%). Numbers and percentages of chemotherapy regimens were as; Adriamycin (Mylan Pharmaceuticals Inc, Teva Pharmaceuticals Inc, Fresenius Kabi USA LLC) – cyclophosphamide (Baxter International Inc, Roxane laboratories Inc, Sandoz Inc) (AC): n=78, (31%), docetaxel (Dr. Reddy’s laboratories Inc, Pfizer Inc, Sandoz Inc, Teva Pharmaceuticals Inc)-AC: n=41, (16.3%), fluorouracil (Accord Healthcare Inc, Fresenius Kabi USA LLC)-epirubicin (amnial Pharmaceuticals Inc, Global Pharmaceuticals Inc, Mylan Pharmaceuticals Inc) -cyclophosphamide (FEC): n=58, (23%), AC or FEC and taxane: 75, (29.7%) respectively. Tamoxifen for pre-perimenopausal and aromatase inhibitors for post-menopausal women were used as hormonal treatments and mean duration of hormonal treatment was 47.34±15.32 months (range 13–120).
Patients who did not receive OST were older than patients who received OST [p=0.004 ≤35 vs. >35; 50 (19.8%)/152 (81.2%) and 45 (32.8%)/92 (67.2%)], lower hormone receptor positivity [p<0.001, (+/−) 181 (71.8%)/71 (28.2%) and 134 (97.8%)/3 (2.2%), respectively], and higher histologic grade [p=0.001, grade (1/2.3) 84 (33.3%)/168 (66.7%) and 68 (50%)/68 (50%), respectively].
Two hundred fifty-two patients received chemotherapy alone without OST. Patients with CIA were older comparing without CIA [p<0.001, ≤35 vs. >35; 12 (8.3%)/133 (91.7%) and 38 (35.5%)/69 (65.5%) respectively]. They had lower histologic grade [p<0.001, grade (1/2.3) 61 (42.1%)/84 (57.9%) and 23 (21.5%)/84 (78.5%), respectively], and had higher hormone receptor positivity [p=0.026, (+/−) 112 (78.2%)/33 (21.8%) and 69 (64.5%)/38 (35.5%), respectively]. In addition, more patients were treated with axillary dissection [p<0.023, AD (−/+) 46 (31.7%)/99 (68.3%) and 49 (45.8%)/58 (54.2%), respectively] (Table 1).
Table 1.
Comparison of patient’s characteristics between CIA and non-CIA in the subgroup of patients treated with chemotherapy without OST
| N (total)=252 | CIA (−) n (%) | CIA (+) n (%) | p |
|---|---|---|---|
| age (years) | |||
| ≤35 n =50 | 38 (35.5%) | 12 (8.3%)/ | |
| >35 n=202 | 69 (64.5%) | 133 (91.7 %) | p<0.001 |
| Molecular subtype | |||
| Luminal A n=80 | 28 (26.2 %) | 52 (35.8 %) | |
| Luminal B n=99 | 41 (38.3%) | 58 (40 %) | |
| Her 2 (+) n=26 | 11 (10.3%) | 15 (10.3%) | |
| Triple (−) n=48 | 27 (25.2%) | 21 (14.4%) | p<0.001 |
| Surgery | |||
| BCS n=163 | 75(70.1%) | 88 (60.6%) | |
| MRM) n=89 | 32 (29.9%) | 57 (39.4%) | p=0.113 |
| LVI (−) n=120 | 52 (48.6%)/ | 68 (47.9%)/ | |
| LVI (+) n=132 | 55 (51.4%) | 77 (53.1%) | P=0.829 |
| Multicentricity/multifocality | |||
| None n=181 | 74 (69.2%)/ | 107 (73.8%)/ | |
| Yes n=71 | 33 (31.8%) | 38 (26.2%) | p=0.419 |
| Axillary dissection | |||
| None n=95 | 49 (45.8%)/ | 46 (31.7%)/ | |
| Yes n=157 | 58 (54.2%) | 99 (68.3%) | p=0.023 |
| HER - 2 | |||
| Negative n=186 | 77 (73.3%)/ | 109 (75.2%)/ | |
| Positive n=66 | 30 (26.7%) | 36 (24.8%) | p=0.722 |
| HR | |||
| Positive n=186 | 74 (71.8%)/ | 112 (78.2%)/ | |
| Negative n=66 | 33 (28.2%) | 33 (22.8%) | p=0.026 |
| CT | |||
| Taxane (+) n=114 | 45 (42.1%)/ | 69 (48.3%)/ | |
| Taxane (−) n=138 | 62 (57.9%) | 76 (51.7%) | p=0.330 |
| RT | |||
| No n=26 | 8 (7.5%)/ | 18 (12.4%)/ | |
| Yes n=226 | 99 (82.5%) | 127 (87.6 %) | p=0.203 |
| Pathological stage | |||
| 1 n=68 | 31(29%)/ | 37 (25.5%)/ | |
| 2+3 n=184 | 76 (71%) | 108 (74.5%) | p=0.541 |
| Histological Grade | |||
| 1/n= 84 | 23 (21.5%)/ | 61 (42.1%)/ | |
| 2+3 n=168 | 84(78.5%) | 84 (57.9%) | p=0.001 |
| Lymph Node | |||
| Positive n=123 | 58 (54.2%)/ | 65 (44.8%)/ | |
| Negative n=129 | 49 (45.8%) | 80 (55.2%) | p=0.141 |
OA: over ablation; CT: chemotherapy; LVI: lymphovascular invasion; RT: radiotherapy
Patients with hormone receptor positive (+) were observed to have less taxane regimen, better survival, less lymph node positivity, and more amenorrheic development (Table 2).
Table 2.
Comparison of patient’s characteristics between HR (+) and HR (−) in the subgroup of patients treated with chemotherapy without OST
| n(total)= 252 | Hormone receptor (+) n=179 (71%) |
Hormone receptor (−) n=73 (29%) |
p |
|---|---|---|---|
| age (years) | |||
| ≤35 n=50 | 34 (18.8%) | 16 (22.5 %)/ | |
| >35 n=202 | 147 (81.2%) | 55 (77.5 %) | p=0.502 |
| Surgery | |||
| BCS n=162 | 118 (65.2%) | 44 (62.9 %) | |
| MRM n=88 | 63 (34.8 %) | 26 (37.1 %) | p=0.529 |
| LVI (−) n=167 | 118 (65.2 %)/ | 49 (69%)/ | |
| LVI (+) n= 85 | 63 (34.8 %) | 22 (31 %) | p=0.564 |
| Multicentricity/multifocality | |||
| None n=181 | 125 (69 %)/ | 56 (78.9 %)/ | |
| Yes n=71 | 56 (31 %) | 15 (21.1%) | p=0.119 |
| Axillary dissection | |||
| None n=181 | 64 (35.4 %)/ | 31 (43.7 %)/ | |
| Yes n=157 | 117 (64.6 %) | 40 (56.3 %) | p=0.221 |
| HER - 2 | |||
| Negative n=186 | 138(76 %)/ | 48 (68 %)/ | |
| Positive n=65 | 43 (24 %) | 23 (32 %) | p=0.188 |
| CIA | |||
| Negative n=107 | 69 (38%)/ | 38 (53.5 %)/ | |
| Positive n=145 | 112 (62%) | 33 (46.5 %) | p<0.026 |
| CT | |||
| Taxane (+) n=115 | 73 (40 %)/ | 42 (59%)/ | |
| Taxane (−) n=137 | 108 (60 %) | 29 (41 %) | p=0.010 |
| RT | |||
| No n=26 | 17 (9.4 %)/ | 9 (12.7 %)/ | |
| Yes n=226 | 164 (90.6 %) | 62 (87.3 %) | p=0.441 |
| Pathological stage | |||
| 1 n=68 | 47 (26 %)/ | 21 (29.6 %)/ | |
| 2+3 n=184 | 134 (74 %) | 50 (70.4 %) | p=0.561 |
| Histological Grade | |||
| 1/n=84 | 66 (36.5 %)/ | 18 (25.4 %)/ | |
| 2+3 n=168 | 115 (63.5 %) | 53 (74.6 %) | p=0.092 |
| Lymph Node | |||
| Positive n=123 | 80 (44.2 %)/ | 43 (60.6 %)/ | |
| Negative n=129 | 101 (55.8 %) | 28 (39.4 %) | p=0.019 |
OA: over ablation; CT: chemotherapy; LVI: lymphovascular invasion; RT: radiotherapy
Survival Analyses
At the end of the median follow-up period of 60 months (range, 24–168 months), 95.9% of those with CIA and 89.7% of those without CIA were alive without OST. The difference between the two groups was significant (p=0.042, mean OS: 158.88±3.70 months vs. 135.33±4.66 months) (Table 3, Figure 1).
Table 3.
Five-year survival analysis: patients who developed CIA had significantly higher OS. In the hormone receptor (+) subgroup analysis, 5-year OS was found statistically higher in the group with CIA than in the non-CIA (p=0.036, 97.3% vs. 91.3%, mean: 136.20±5.17 vs. 162.13±3.39). In patients with CIA, OS, DFS, and MFS durations of Luminal A type were significantly longer than luminal B type, but there was no difference in survival durations in patients without CIA (p=0.027 vs 0.074, p=0.023 vs 0.963, p = 0.016 vs 0.911, respectively
| OS/(5 y) p | DFS/(5 y) p | LRFS/(5 y) p | MFS/(5y) p | |
|---|---|---|---|---|
| CIA(−)/CIA(+) | 89.7%/95.9%, p=0.042 | 84.8%/83.2%, p=0.693 | 92.5%/95.1%, p=0.413 | 88.9%/89.0%, p=0.974 |
| CIA(−), HR(+)/HR(−) | 91.3%/86.8%, p=0.424 | 85.5%/78.9%, p=0.368 | 95.7%/86.8%, p=0.064 | 88.4%/89.5%, p=0.885 |
| CIA(+), HR(+)/HR(−) | 97.3%/90.9%, p=0.011 | 85.7%/81.8%, p=0.238 | 96.4%/90.9%, p=0.066 | 89.5%/87.9%, p=0.515 |
| CIA(−), LUMA/LUMB | 96.4%/87.8%, p=0.074 | 82.1%/87.8%, p=0.963 | 92.9%/97.6%, p=0.716 | 85.2%/90.5%, p=0.911 |
| CIA(+), LUMA/LUMB | 100%/94.8%, p=0.027 | 92.3%/81.0%, p=0.023 | 98.1%/94.8%, p=0.215 | 96.2%/84.5%, p=0.016 |
OS: overall survival; DFS: disease-free survival; LRFS: local-recurrence-free survival; DMFS: Distant metastasis-free survival
Figure 1.
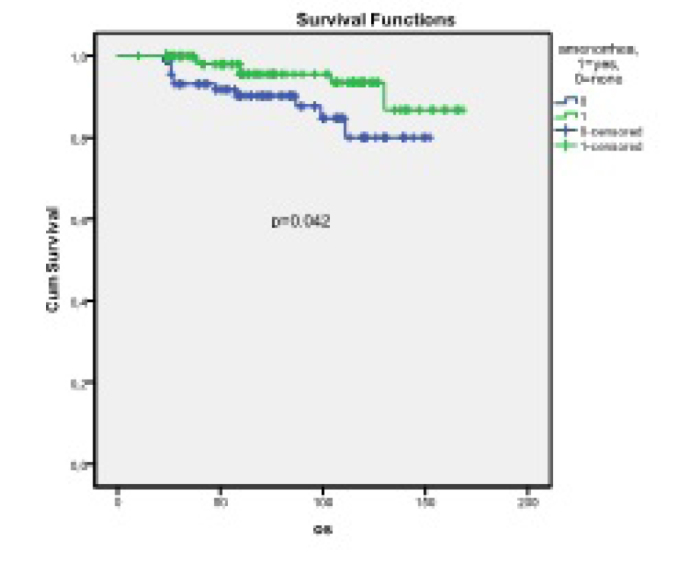
Kaplan-Meier curve of patients with CIA and without CIA who were not treated with OST. At the end of the median follow-up period of 60 months (24–168), 95.9% of patients with CIA and 89.7% of those without CIA were alive without OST. (p=0.042, mean OS: 158.88±3.70 months vs 135.33±4.66 months)
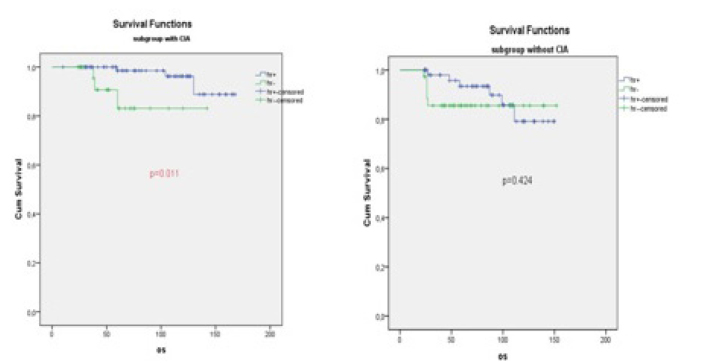
In the CIA (+) subgroup analysis, 5-year OS was found statistically higher in the group with HR (+) than in the HR (−) group (p=0.011, 97.3% vs. 90.9%, mean: 162.13±3.39 vs. 126.16±8.48) (Table 3, Figure 2).
Figure 2.
In the CIA (+) subgroup analysis, 5-year OS was found statistically higher in the group HR (+) than in the HR (−) (p=0.011, 97.3% vs 90.9%, mean: 162.13±3.39 vs 126.16±8.48). In the CIA (−) subgroup analysis, 5-year OS was not significantly different compared with groups with HR (+) and HR(−) (p=0.424: 86.8% vs. /91.3%, mean: 136.2±7.55 vs. 133.72±5.17)
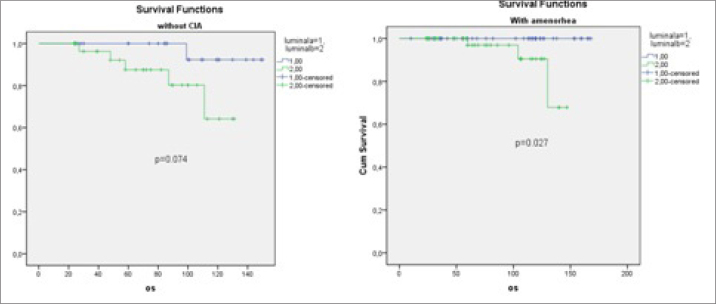
In patients with CIA; OS, DFS, and DMFS durations of luminal A type were significantly longer than luminal B type, but there was no difference in survival durations in patients without CIA (p=0.027 vs. 0.074, p=0.023 vs. 0.963, p=0.016 vs. 0.911, respectively) (Table 3, Figure 3). In the luminal B molecular subtype, a higher taxane-containing chemotherapy regimen was used (49% vs. 28.8%, p=0.006), but there was no significant difference in clinicopathological characteristics in either group.
Figure 3.
In patients with CIA, OS durations of Luminal A type were significantly longer than luminal B type, but there was no difference in survival durations in patients without CIA (p=0.027 vs. 0.074, respectively)
Twenty four percent of the patients (n=95) were ≤35 years old. At the end of the five-year follow-up, 92.1% of patients who were hormone receptor+and 89.5% of those who were hormone receptor - were alive at age ≤35 years (p=0.600, mean OS; 131.4 months vs. 136.3 months). At the end of the five-year follow-up, 97.1% of patients who were hormone receptor + and 89.1% of those who were hormone receptor - were alive at age >35 years (p=0.001, mean OS: 160.0 months vs. 124 months, respectively). Medical ovarian ablation was performed in 47.4% of patients aged 35 years and younger and 68.7% in patients aged >35 years (p=0.004). CIA developed in 24% of patients aged ≤35 years old (n=12) and 65.8% (n=133) of those aged over 35 years without OA (p=0.0001)
The 5-year OS rate of patients who had LN involvement in those who had only chemotherapy without OST tended to be higher than those who had no LN involvement who had CIA (p=0.051; 95% vs. 83.7% (mean: 149.43±5.14 vs. 128.72±7.26). In this group, the 5-year OS rate of patients who had pathologic stage 2–3 with CIA was significantly higher than patients without CIA (p=0.011; 95.4% vs. 85.5%) (mean: 156.72±4.06 vs 129.33±6.11).
There was no difference in 5-year overall survival (OS) between rates of CIA presence and absence in patients who had only chemotherapy without OST in pathologic stage 1 (p=0.450, 94.8% vs. 96.9%, respectively) (p=0.344, respectively).
Multivariate Analysis
Molecular subtypes, surgical procedures (mastectomy - MKC), lymphovascular invasion, MC/MF, advanced pathologic stage (stage 2–3), aged ≤40 years, aged ≤35 years, hormone receptor positivity PR), HER2 positivity, radiotherapy, chemotherapy regimen (with or without taxane, distant metastasis, local recurrence and amenorrhea were assessed using Cox regression analysis of OS supplementation; molecular subtype, multicentricity - multifocal presence and relapse of the disease were independent parameters affecting OS.
Molecular subtypes: p=0.046, HR: 7.375, (95% CI: [1.036–52.412])multicentricity – multifocality: p=0.023, HR: 4.750, (95% CI: [1.240–18.201])
recurrence: p=0.004, HR: 18.348, (95% CI: [2.517–133.765])
Molecular subtype was an independent effector factor on OS in patients without OA in the Cox regression analysis (p=0.008, HR: 33.44, 95% CI: [2.49–448.53]).
Discussion and Conclusion
The prognosis of patients with premenopausal breast cancer at diagnosis is worse than that of postmenopausal women (19). It has been shown that patients with breast cancer under the age of 35 years have a shorter survival time when other prognostic factors are examined than those aged over 35 years (20–22). Breast cancer in the premenopausal stage triple-negative and HER2-positive more often than in postmenopausal women (23, 24). However, patients with a hormone receptor-positive (luminal) molecular subtype breast cancer below the age of 40 have worse survival times than those aged over 40 years. In HER 2-positive patients, there was a negative trend in patients aged 40 years or younger with breast cancer, but there was no difference in prognostic characteristics between the two age groups in triple-negative molecular subtypes (25). In our study, the OS duration in the luminal A molecular subtype was significantly worse among women aged ≤35 years than those aged >35 years, whereas there was no significant difference between the two age groups in the luminal B subgroup (luminal A; p=0.039, 5-y OS: 92.9% vs. 100% and luminal B: p=0.898, 5-y OS 88.9% vs. 92.6%).
Adjuvant chemotherapy provides a significant survival advantage in patients with premenopausal breast cancer (3, 4). Studies have shown that chemotherapy-induced amenorrhea is more prominent in patients who are hormone receptor-positive, but independent of hormone receptor status (4–7, 9). Amenorrhea caused by adjuvant chemotherapy has been described in various studies as amenorrhea that develops for at least 3–12 months within 12 months after the completion of chemotherapy (9). In this study, amenorrhea lasting at least six months was accepted as CIA. Despite of survival benefit of transient amenorrhea due to chemotherapy resumption of menstruation after CIA is not associated poor prognosis (26). According to the results of our study, amenorrhea due to adjuvant chemotherapy showed a significant OS advantage in patients with premenopausal breast cancer who did not undergo OST (p=0.042). In the CIA (+) subgroup analysis, 5-year OS was found statistically higher in the group with HR (+) than in the HR (−) group (p=0.011, 97.3% vs. 90.9%, mean: 162.13±3.39 vs. 126.16±8.48). However, in patients without CIA there was no prognostic benefit between HR (+) and HR (−) groups (p=0.424). Despite the fact that CIA contributes positively to survival, the number of prospective randomized clinical trials in this area is very small. The National Surgical Adjuvant Breast and Bowel Project (NSABP-30) study showed significant survival advantage with CIA independently of estrogen receptor status (NSABP-30), but subsequent analysis of this study (4) and the International Breast Cancer Study Group (IBCSG 13–93, VI, VIII) study indicated that the CIA-related survival effect was limited in patients who were hormone receptor positive (5, 27, 28). These literature data are in accordance with the results obtained in our study.
Some studies have pointed to the fact that CIA is age-dependent (20–71% for 40 years and lower, 40–100% for those aged over 40 years (20, 21, 23). Similarly, for our study, the rate of CIA in those aged >35 years (n=133, 65.7%)) higher than in those ≤35 years of age (n=12, 24%), (p=0.001)]. We found that overall survival in the ≤35 age group did not significantly contribute to overall survival compared with patients aged >35 years in terms of the prognostic impact of CIA. There are conflicting data on the age-related prognostic impact of CIA in the literature, for example, Bonadonna G. et al (29) showed that CIA contributed to survival at young age, and similar findings were reported in the IBCSG-6 trial (5). However, in the NSABP B-30 trial, it was emphasized that amenorrhea contributed to survival in all age groups (4). When all patients participating in the study were evaluated together, hormone receptor positivity significantly extended overall survival, but this increase was not significant in the ≤35 age group. Along with the development of more CIA at older ages, the effect of both hormone receptor positivity and CIA development on survival time at young age, especially in those aged ≤35 years, was not shown in our study in accordance with similar examples in the literature. In patients with a high risk of distant metastasis according to the results of Soft and TEXT studies and in the ≤35 age group, treatment with OST supplementation provides a significant benefit (30). In this study, despite less frequent amenorrhea due to chemotherapy in the ≤35 age group, patients with amenorrhea with medical ovarian ablation and/or chemotherapy showed no survival advantage compared with those who did not develop amenorrhea. This result can be explained by the fact that the median OST duration of our study was shorter than Soft and Text studies (24 vs. 60 months).
Although the survival relationship in patients with hormone receptor-positive disease is better known, the effect of chemotherapy-induced amenorrhea on survival in patients with different molecular subtypes is unknown (9, 31). The most important feature of our study is the separation of patients into molecular subtypes according to St. Gallen 2013 criteria and the investigation of the overall survival contribution of amenorrhea due to chemotherapy in patients with these different molecular subtypes. In patients with amenorrhoea who had luminal A molecular subtypes had longer survival times than those with luminal B; patients without amenorrhea did not show such a difference between the luminal types (p=0.027 vs. 0.074 respectively). The fact that hormonal therapy is more effective in luminal A molecular subtype may explain the better survival outcome of luminal A in CIA, which is actually a side effect of chemotherapy. In IBCSG 15–95 and high dose Dutch studies, it has been found that this effect is greater than the logical size (32).
Most studies investigating the effects of CIA on survival suggest that amenorrhea has the advantage of survival in patients with lymph node involvement (3, 5, 29, 33). Consistent with these data, the overall survival time in our study was significantly longer in patients with CIA and in more advanced stage at diagnosis compared with patients who had CIA and early-stage disease (p<0.001). Studies showing the relationship between chemotherapy regimens and amenorrhea have different results (9). Most studies show that cyclophosphamide-containing regimens cause more amenorrhea (28, 31). Roche et al. (34) reported that the addition of treatment to taxanes does not increase the frequency of amenorrhea development. In our study, amenorrhea did not increase with chemotherapy-including taxane.
The limiting aspects of our work are that it was retrospective and that the analysis was based on a heterogeneous group of patients. The results of this age group should be evaluated carefully because of the low number of patients aged under thirty-five years. A more comprehensive assessment of data due to the inclusion of patients from a single center can be considered as one of the positive aspects of our study.
In conclusion, the development of amenorrhea due to chemotherapy in patients with early-stage breast cancer and hormone receptor positivity significantly increases survival. The survival advantage of luminal A was higher than in patients who had a luminal B molecular subtype. The contribution of chemotherapy-induced amenorrhea is more pronounced in patients with lymph node involvement.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul Bilim University (44140529/2017-71).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.Ö., Ç.O., G.A.; Design - V.Ö., Ç.O., Y.E., G.A., K.N.P.; Supervision - V.Ö., Ç.O., Y.E., K.N.P.; Resources - V.Ö., Ç.O., G.A., K.N.P.; Materials - V.Ö., Ç.O., C.T., K.N.P.; Data Collection and/or Processing - Ç.O., F.E., F.A., K.N.P., G.A., F.A.; Analysis and/or Interpretation - V.Ö., Ç.O., Y.E., F.A., Ü.İ.K.; Literature Search - Ç.O., D.S.; Writing Manuscript - Ç.O., K.N.P., V.Ö., F.Ç., Z.İ.E.; Critical Review - V.Ö., Ç.O., S.İ., G.A.; Other - A.Ö., T.D., S.İ.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Ozmen V. Breast Cancer in Turkey: Clinical and Histopathological Characteristics (Analysis of 13.240 Patients) J Breast Health. 2014;10:98–105. doi: 10.5152/tjbh.2014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. [Accessed 3 Dec 2013]. Available: http://seer.cancer.gov/csr/1975_2010/sections.
- 3.Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, De Lena M, Tancini G, Bajetta E, Musumeci R, Veronesi U. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, Jeong JH, Geyer CE, Jr, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, Rastogi P, Livingston RB, Perez EA, Mamounas EP, Land SR, Ganz PA, Wolmark N. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagani O, O’Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, Lindtner J, Collins J, Crivellari D, Coates A, Cavalli F, Thürlimann B, Simoncini E, Fey M, Price K, Senn HJ. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: Results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–640. doi: 10.1016/S0959-8049(97)10036-3. [DOI] [PubMed] [Google Scholar]
- 6.Parulekar WR, Day AG, Ottaway JA, Shepherd LE, Trudeau ME, Bramwell V, Levine M, Pritchard KI National Cancer Institute of Canada Clinical Trials Group. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: Analysis of a National Cancer Institute of Canada Clinical Trials Group Study—NCIC CTG MA.5. J Clin Oncol. 2005;23:6002–6008. doi: 10.1200/JCO.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 7.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Liu J, Chen K, Li S, Wang Y, Yang Y, Deng H, Jia W, Rao N, Liu Q, Su F. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113–128. doi: 10.1007/s10549-014-2914-x. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group. Ovarian ablation in early breast cancer: overview of the randomized trials. Lancet. 1996;348:1189–1196. doi: 10.1016/S0140-6736(96)05023-4. [DOI] [PubMed] [Google Scholar]
- 11.Adjuvant Breast Cancer Trials Collaborative Group. Ovarian Ablation or Suppression in Premenopausal Early Breast Cancer: Results From the International Adjuvant Breast Cancer Ovarian Ablation or Suppression Randomized Trial. J Natl Cancer Inst. 2007;99:516–525. doi: 10.1093/jnci/djk109. [DOI] [PubMed] [Google Scholar]
- 12.Boccardo F, Rubagotti A, Perrotta A, Amoroso D, Balestrero M, De Matteis A, Zola P, Sismondi P, Francini G, Petrioli R. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol. 1994;5:337–342. doi: 10.1093/oxfordjournals.annonc.a058837. [DOI] [PubMed] [Google Scholar]
- 13.Taylor CW, Green S, Dalton WS, Martino S, Rector D, Ingle JN, Robert NJ, Budd GT, Paradelo JC, Natale RB, Bearden JD, Mailliard JA, Osborne CK. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an Intergroup Study. J Clin Oncol. 1998;16:994–999. doi: 10.1200/JCO.1998.16.3.994. [DOI] [PubMed] [Google Scholar]
- 14.International Breast Cancer Study Group (IBCSG) Castiglione-Gertsch M, O’Neill A, Price KN, Goldhirsch A, Coates AS, Colleoni M, Nasi ML, Bonetti M, Gelber RD. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann M, Jonat W, Blamey R, Cuzick J, Namer M, Fogelman I, de Haes JC, Schumacher M, Sauerbrei W Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists’ Group. Survival analyses from the ZEBRA study: Goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cance. 2003;39:1711–1717. doi: 10.1016/S0959-8049(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Wu SG, Wang JJ, Sun JY, Li FY, Lin Q, Lin HX, He ZY. Ovarian Ablation Using Goserelin Improves Survival of Premenopausal Patients with Stage II/III Hormone Receptor-Positive Breast Cancer without Chemotherapy-Induced Amenorrhea. Cancer Res Treat. 2015;47:55–63. doi: 10.4143/crt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JG, Jiang YD, Zhang CH, Pang D, Zhang M, Wang YB, Xue WN, Sun Q. Clinicopathologic characteristics and prognosis of young patients with breast cancer. Breast. 2011;20:370–372. doi: 10.1016/j.breast.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng R, Wang S, Shi Y, Liu D, Teng X, Qin T, Zeng Y, Yuan Z. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011;20:568–573. doi: 10.1016/j.breast.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Eccles BK, Copson ER, Cutress RI, Maishman T, Altman DG, Simmonds P, Gerty SM, Durcan L, Stanton L, Eccles DM POSH Study Steering Group. Family history and outcome of young patients with breast cancer in the UK (POSH study) Br J Surg. 2015;102:924–935. doi: 10.1002/bjs.9816. https://eprints.soton.ac.uk/380822/ [DOI] [PubMed] [Google Scholar]
- 23.Brennan M, French J, Houssami N, Kirk J, Boyages J. Breast cancer in young women. Aust Fam Physician. 2005;34:851–855. [PubMed] [Google Scholar]
- 24.Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-Demore N, Perou CM. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18–20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Tamimi RM. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 26.Jeon SJ, Lee JI, Jeon MJ, Lee M. Prognostic effects of adjuvant chemotherapy-induced amenorrhea and subsequent resumption of menstruation for premenopausal breast cancer patients. Medicine (Baltimore) 2016;95:e3301. doi: 10.1097/MD.0000000000003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Breast Cancer Study Group. Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, Price KN, Coates AS, Gelber RD. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson P, Sun Z, Braun D, Price KN, Castiglione-Gertsch M, Rabaglio M, Gelber RD, Crivellari D, Collins J, Murray E, Zaman K, Colleoni M, Gusterson BA, Viale G, Regan MM, Coates AS, Goldhirsch A. Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011;22:2216–2226. doi: 10.1093/annonc/mdq735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 30.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE, Jr, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q, Yin W, Du Y, Shen Z, Lu J. Prognostic impact of chemotherapy-induced amenorrhea on premenopausal breast cancer: meta-analysis of the literatüre. Menopause. 2015;22:1091–1097. doi: 10.1097/GME.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coates AS, Colleoni M, Goldhirsch A. Adjuvant Chemotherapy Useful for Women With Luminal A Breast Cancer? J Clin Oncol. 2012;30:1260–1263. doi: 10.1200/JCO.2011.37.7879. [DOI] [PubMed] [Google Scholar]
- 33.Ganz PA, Land SR, Geyer CE, Jr, Cecchini RS, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff JA, Vogel VG, Erban JK, Livingston RB, Perez EA, Mamounas EP, Wolmark N, Swain SM. Menstrual History and Quality-of-Life Outcomes in Women With Node-Positive Breast Cancer Treated With Adjuvant Therapy on the NSABP B-30 Trial. J Clin Oncol. 2011;29:1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roché H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, Symann M, Kerbrat P, Soulié P, Eichler F, Viens P, Monnier A, Vindevoghel A, Campone M, Goudier MJ, Bonneterre J, Ferrero JM, Martin AL, Genève J, Asselain B. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNLCC PACS 01 trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]