Abstract
Kallikrein-related peptidase 6 (Klk6) is the most abundant serine proteinase in the adult central nervous system (CNS), yet we know little regarding its physiological roles or mechanisms of action. Levels of Klk6 in the extracellular environment are dynamically regulated in CNS injury and disease positioning this secreted enzyme to affect cell behavior by potential receptor dependent and independent mechanisms. Here we show that recombinant Klk6 evokes increases in intracellular Ca2+ in primary astrocyte monolayer cultures through activation of proteinase activated receptor 1 (PAR1). In addition, Klk6 promoted a condensation of astrocyte cortical actin leading to an elongated stellate shape and multicellular aggregation in a manner that was dependent on the presence of either PAR1 or PAR2. Klk6-evoked changes in astrocyte shape were accompanied by translocation of β-catenin from the plasma membrane to the cytoplasm. These data are exciting because they demonstrate that Klk6 can influence astrocyte plasticity through receptor-dependent mechanisms. Furthermore, this study expands our understanding of the mechanisms by which kallikreins can contribute to neural homeostasis and remodeling and point to both PAR1 and PAR2 as new therapeutic targets to modulate astrocyte form and function.
Keywords: Astrogliosis, Beta Catenin, Calcium signaling, Kallikrein, Neurosin, Astrogliosis
Introduction
Astrocytes are chemically excitable cells that undergo dramatic changes in cell shape in response to environmental cues. Emerging studies suggest these changes play essential roles in regulating central nervous system (CNS) physiology and in the progression of pathologies, including the response of the brain and spinal cord to injury (Pekny et al. 2016). Kallikrein-related peptidase 6 (kallikrein 6 (Klk6), also referred to as Zyme (Little et al. 1997), Protease M (Anisowicz et al. 1996), Neurosin (Yamashiro et al. 1997) or myelencephalon specific protease (MSP) (Scarisbrick et al. 1996; Scarisbrick et al. 1997; Scarisbrick et al. 2012a), is well positioned to participate as a chemical regulator of astrocyte physiology and pathophysiology. First, Klk6 is the most abundant serine proteinase in the adult CNS and its levels are dynamically altered in the context of CNS injury and disease (Scarisbrick 2012). In the human CNS, KLK6 levels are increased in active MS lesions (Scarisbrick et al. 2002), after traumatic spinal cord injury SCI (Scarisbrick et al. 2006; Radulovic et al. 2013; Radulovic et al. 2015) and in high grade astrocytoma (Drucker et al. 2013; Drucker et al. 2015), but decreased in Alzheimers (Little et al. 1997; Diamandis et al. 2000; Ogawa et al. 2000; Zarghooni et al. 2002; Ashby et al. 2010) and Parkinsons disease (Iwata et al. 2003; Spencer et al. 2013; Spencer et al. 2015; Pampalakis et al. 2017). Indeed, astrocytes express very low levels of Klk6 in the intact CNS (Scarisbrick et al. 1997; Scarisbrick et al. 2000), however astrocyte Klk6 is up regulated in response to a variety of insults. Induction of Klk6 expression in astrocytes is observed in several experimental neural injury models, including traumatic injury (Scarisbrick et al. 2006), excitotoxicity (Scarisbrick et al. 1997), and in neuroinflammatory conditions (Scarisbrick et al. 2002). Pro-inflammatory cytokines such as interleukin 6 (IL-6) also trigger expression of astrocyte Klk6 (Vandell et al. 2008; Radulovic et al. 2015; Radulovic et al. 2016).
Our understanding of the physiological actions of Klk6 in astrocyte biology and pathology are currently very limited. Prior studies demonstrate astrocytes treated with Klk6 undergo a rapid transition from an epithelioid to a stellate morphology, increase IL-6 expression and secretion (Scarisbrick et al., 2012), and show activation of the mitogen-activated protein kinases (MAPK) (Vandell et al., 2008) and signal transducer and activator of transcription 3 (STAT3) (Radulovic et al. 2015; Radulovic et al. 2016) signaling pathways. KLK6 also promotes survival and resistance of astrocytoma cell lines to irradiation and temozolomide with higher tumor KLK6 levels associated with reduced patient survival (Drucker et al. 2013; Drucker et al. 2015).
As a secreted proteinase considerable attention has been focused on the actions of KLK6 as a modifier of extracellular matrix proteins (see (Borgono et al. 2004; Sotiropoulou et al. 2010; Prassas et al. 2015) for review). For example, studies show heat-denatured collagen, laminin, fibronectin and aggrecan are all hydrolyzed by Klk6 (Bernett et al. 2002; Blaber et al. 2002; Scarisbrick et al. 2006). Also, KLK6 cleaves several disease relevant proteins, such as myelin basic protein and myelin oligodendrocyte glycoprotein (Bernett et al. 2002; Blaber et al. 2002; Scarisbrick et al. 2002; Blaber et al. 2004; Angelo et al. 2006), amyloid precursor protein (Little et al. 1997; Magklara et al. 2003), and α-syneuclein (Iwata et al. 2003; Kasai et al. 2008; Tatebe et al. 2010; Spencer et al. 2015; Pampalakis et al. 2017). In addition, it is now clear that certain kallikrein-related peptidases, including Klk6, can site-specifically cleave the extracellular N terminal domain of a subset of proteinase activated receptors (PARs) to alter intracellular signaling and cell behavior directly in a hormone or cytokine-like fashion (Angelo et al. 2006; Oikonomopoulou et al. 2006a; Oikonomopoulou et al. 2006b; Vandell et al. 2008; Scarisbrick et al. 2011; Scarisbrick et al. 2012b; Radulovic et al. 2015; Radulovic et al. 2016).
Proteinase activated receptors have seven transmembrane helices coupled to intracellular heterotrimeric G proteins with proteolytic activation unmasking a new tethered peptide sequence that folds back onto the PAR serving as a ligand to elicit intracellular signaling. There are four proteinase activated receptors (PAR1–4) and each is expressed at significant levels across the brain and spinal cord (Citron et al. 2000; Wang et al. 2002; Junge et al. 2003; Noorbakhsh et al. 2003; Junge et al. 2004; Nicole et al. 2005; Vandell et al. 2008; Yoon et al. 2013; Radulovic et al. 2015; Allen et al. 2016; Radulovic et al. 2016). Expression of all 4 PARs has been demonstrated in purified cultures of murine cortical neurons and purified astrocytes as well as the Neu7 astrocyte cell line (Vandell et al. 2008). Growing evidence suggests that targeting the activity of select PARs, improves neurological outcomes in experimental models of ischemia (Junge et al. 2003; Rajput et al. 2014), in the experimental autoimmune encephalomyelitis (Noorbakhsh et al. 2006) and after SCI (Radulovic et al. 2015; Radulovic et al. 2016). Of the several kallikreins examined to date there is evidence that KLK5, KLK6, KLK7 and KLK14 (Angelo et al. 2006; Oikonomopoulou et al. 2006a; Oikonomopoulou et al. 2006b; Vandell et al. 2008), but not KLK8 (Ramachandran et al. 2012), or KLK1 (Vandell et al. 2008), activate PARs. With regard to KLK6, the recombinant enzyme mobilizes Ca2+ in rat v-K-ras transformed Normal Rat Kidney PAR2 over-expressing cells and mediates aortic ring relaxation in a PAR2-dependent fashion (Oikonomopoulou et al. 2006a; Oikonomopoulou et al. 2006b). KLK6 also cleaves thereby activating PAR1 in neurons and both PAR1 and PAR2 in the Neu7 astrocyte cell line eliciting increases in intracellular Ca2+ and differential signaling of MAPK family members and protein kinase B (AKT) (Vandell et al. 2008). KLK6 also increases B-cell lymphoma 2 (Bcl2) family member signaling in a PAR1 dependent manner to promote survival of human and murine lymphocytes (Scarisbrick et al. 2011). The ability of Klk6 to promote resistance of glioblastoma cells to irradiation and temozolomide is also mediated, at least in part, through PAR1 (Drucker et al. 2013). Taken together, these prior studies suggest KLK6 is positioned to regulate cell physiology and pathophysiological responses through receptor dependent mechanisms.
Although we previously demonstrated Klk6 triggers of reactive astrogliosis (Vandell et al. 2008; Scarisbrick et al. 2012a), the extent to which this relies on receptor-dependent mechanisms has not been clarified. Understanding the mechanisms mediating KLK6 signaling in primary astrocytes is highly significant since mice lacking PAR1 or PAR2 show dramatic reductions in astroglial scar formation and significant improvements in motor outcomes after traumatic SCI (Radulovic et al. 2015; Radulovic et al. 2016). In the current study, we use a combination of pharmacologic and genetic approaches to define the roles of PAR1 and PAR2 in regulating the Klk6-evoked Ca2+ signaling and cell shape changes in primary murine cortical astrocytes.
Results
Kallikrein-related peptidase 6 elicits Ca2+ signaling in astrocytes by activation of PAR1
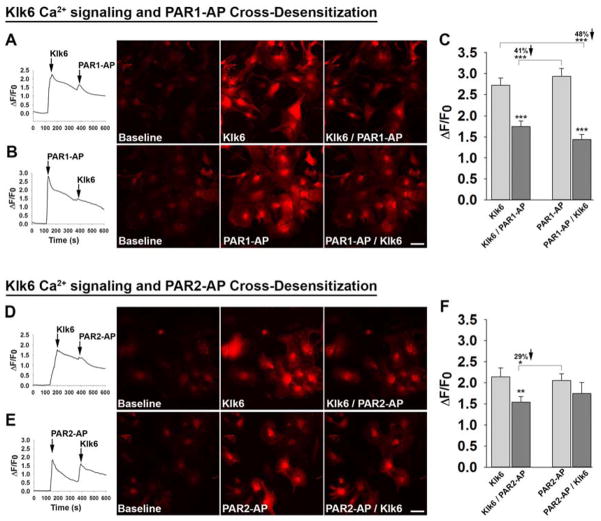
The ability of Klk6 to evoke Ca2+ signaling in primary murine cortical astrocytes was assessed by determining Klk6-elicited changes in fluorescence intensity in Rhod-3 loaded astrocytes (Figure 1A to F). Klk6 (150 nM) produced a greater than 2-fold increase in fluorescence intensity over that seen at baseline. Similarly, application of PAR1-AP (40 μM) or PAR2-AP (200 μM), also elicited greater than a 2-fold increase in intracellular Ca2+ levels (P < 0.001, Students t-test). Increases in fluorescence intensity caused by each agonist were observed within a few seconds of application and occurred in the majority of cells imaged in each field.
Figure 1. Kallikrein related peptidase 6-mediated Ca2+ signaling in primary astrocytes is regulated in part by activation of PAR1.
Traces, photomicrographs, and histograms show fluorescence intensity measured in Rhod-3 loaded murine astrocytes at baseline and in response to recombinant Klk6 (150 nM), a PAR1-activating peptide (PAR1-AP, 40 μM), or a PAR2-activating peptide (PAR2-AP, 100 μM), applied alone or sequentially. (A to F) Application of Klk6 or PAR-APs alone elicited a rapid increase in intracellular Ca2+ expressed as change in fluorescence intensity (ΔF) over baseline intensity (F0), expressed as ΔF/F0 = [(F – F0)/F0]. Subsequent application of either PAR1-AP (A to C), or PAR2-AP (D to F), 240 s later resulted in significantly lower ΔF/F0. Demonstrating that Klk6-elicited Ca2+ signaling occurs primarily down stream of PAR1, reductions in ΔF/F0 were observed when astrocytes were first treated with PAR1-AP prior to application of Klk6 240 s later (B and C). Treatment of astrocytes with PAR2-AP did not significantly alter subsequent Klk6-elicited Ca2+ responses (E and F). These receptor cross desensitization experiments demonstrate that PAR1 plays an important role in Klk6-induced Ca2+ signaling in astrocytes. (*P < 0.05, **P < 0.01, *** P < 0.001, Students t-test). Scale bar = 50 μm.
To determine the extent to which Klk6-gated intracellular Ca2+ increases specifically by activation of astrocyte PAR1, receptor cross-desensitization experiments were performed (Figure 1A to C). First, application of PAR1-AP 240 s after the application of Klk6 resulted in a 41% lower Ca2+ response relative to that seen with PAR1-AP alone (Figure 1A and C, P < 0.001, Students t-test). Similarly, when Klk6 was applied 240 s after PAR1-AP, a 48% reduction in Ca2+signaling was observed (Figure 1B and C, P < 0.001, Students t-test). Reductions in signaling after the application of a second agonist are referred to as receptor cross-desensitization with results here suggesting that Klk6 evokes Ca2+ signaling in primary astrocytes at least in part by activation of PAR1.
To determine whether Klk6-mediated activation of PAR2 may also be involved in Klk6-gated intracellular Ca2+ increases in primary astrocytes, receptor cross-desensitization experiments were performed parallel to those described for PAR1 (Figure 1D to F). First, application of PAR2-AP 240 s after the application of Klk6 resulted in a 29% lower Ca2+ response relative to that seen with PAR2-AP alone (Figure 1D and F, P = 0.04, Students t-test). Similarly, when Klk6 was applied 240 s after PAR2-AP, a 19% reduction in Ca2+signaling was observed, although this reduction did not reach the level of statistical significance (Figure 1E and F). The extent of receptor desensitization observed suggests that Klk6 can cleave and desensitize PAR2. These current findings also suggest that application of PAR2-AP alone is not sufficient to desensitize astrocytes to Klk6-evoked increases in intracellular Ca2+ signaling.
Kallikrein-related peptidase 6 promotes astrocyte stellation, aggregation and translocation of β-Catenin in a PAR1 and PAR2-dependent manner
Klk6-PAR Astrocyte Stellation
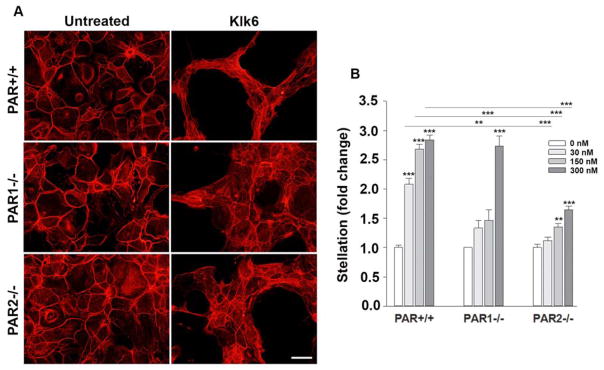
Klk6 is up regulated in astrocytes in a wide variety of CNS insults, including SCI, multiple sclerosis and glioblastoma (Scarisbrick et al. 1997; Scarisbrick et al. 2002; Scarisbrick et al. 2012b; Drucker et al. 2013). Treatment of primary murine cortical astrocyte cultures with Klk6 promotes a rapid change in cell shape, including adoption of an elongated stellate morphology and evident multicellular aggregation (Scarisbrick et al. 2012b). To determine if these rapid alterations in cell shape and association may be mediated by PAR-dependent mechanisms, we quantified the ability of Klk6 to elicit astrocyte stellation, aggregation, and β-catenin translocation in monolayer cultures derived from wild type (PAR+/+) mice or from those in which PAR1 or PAR2 was genetically deleted (Figures 2 to 4). First, in wild type astrocytes, Klk6 promoted a dose-dependent increase in stellation, with 30, 150 and 300 nM of Klk6 driving greater than 2-fold increases (P < 0.001, NK, Figure 2A and B). In astrocytes lacking PAR1, only the highest levels of Klk6, that is 300 nM, were effective in promoting stellation. In the absence of PAR1, stellation induced by 30 or 150 nM Klk6 was reduced by 36% and by 46%, respectively (P ≤ 0.002, NK). In astrocytes lacking PAR2, both 150 and 300 nM of Klk6 promoted stellation (P ≤ 0.007, NK), but the magnitude of stellation in each case was reduced by 40–50%. 30 nM of Klk6 did not promote stellation of astrocytes lacking PAR2. The extent of stellation at baseline did not differ between astrocytes derived wild type mice or those with genetic PAR1 or PAR2 knockout. Taken together, these results suggest that Klk6-mediated alterations in astrocyte stellation occur by mechanisms that depend on the presence of either PAR1 or PAR2.
Figure 2. Kallikrein 6 promotes astrocyte stellation in a PAR-dependent manner.
Photomicrographs and histograms show that recombinant Klk6 promotes a loss of cortical actin filaments in primary astrocytes and transition toward a stellate morphology. The ability of Klk6 to promote astrocyte stellation was significantly reduced in astrocyte cultures derived from PAR1−/− or PAR2−/− mice relative to their wild type counterparts (PAR+/+). Actin filaments were visualized using Cy3-conjugated Phalloidin. See Figure 3 for immunochemical appearance of β-catenin and Figure 4 for nuclear DAPI staining in the same microscopic fields. In Fig. 2–4, asterisks above columns refer to significant changes compared to 0 nM and asterisks above the lines refer to significant changes observed in PAR1−/− or PAR2−/− compared to PAR+/+. (**P < 0.01, *** P < 0.001, NK). Scale bar = 50 μm.
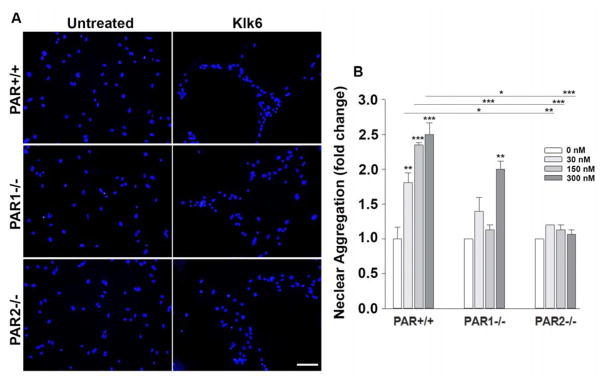
Figure 4. Kallikrein 6 promotes astrocyte aggregation in a PAR-dependent manner.
Photomicrographs and histograms show that recombinant Klk6 elicits cell aggregation. Aggregation was quantified by measuring the distance between DAPI stained nuclei. Klk6-induced cell aggregation was significantly reduced in astrocyte cultures derived from PAR1−/− or PAR2−/− mice relative to their wild type counterparts (PAR+/+). DAPI stained nuclei were visualized along with actin using Cy3-conjugated Phalloidin (Figure 2) and immunofluorescence for beta catenin (Figure 3). (*P < 0.05, **P < 0.01, *** P < 0.001, NK). Scale bar = 50 μm.
Klk6-PAR astrocyte β-catenin translocation
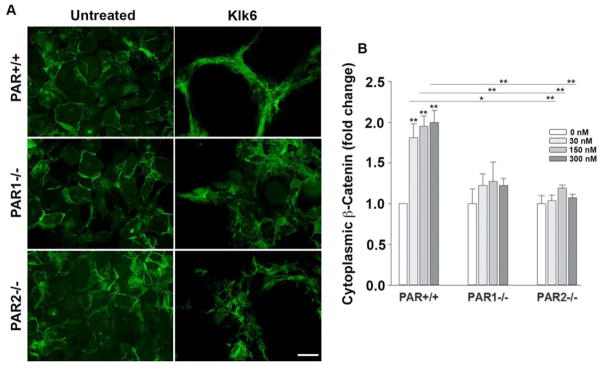
β-catenin plays an important role in regulating cell adhesion and shape and therefore we examined its appearance in astrocytes prior to and after Klk6 treatment, and any role of PAR in the changes observed. Klk6 promoted approximately a 2-fold loss of β-catenin from the astrocyte plasma membrane and an accumulation in the cytoplasm in wild type astrocytes at all concentrations examined that is, 30, 150 and 300 nM (Figure 3, P ≤ 0.003, NK). The absence of PAR1 or PAR2 completely blocked Klk6-mediated β-catenin translocation from the astrocyte surface to the cytoplasm. These findings highlight a novel role for Klk6 in regulation of β-catenin and indicate that both PAR1 and PAR2 play a critical role in this process, such that the absence of either receptor completely prevents translocation.
Figure 3. Kallikrein 6 promotes translocation of β-Catenin from the astrocyte plasma membrane to the cytoplasm in a PAR-dependent manner.
Photomicrographs and histograms show that recombinant Klk6 elicits a change in the appearance of immunoreactivity for β-Catenin from the astrocyte surface to a cytoplasmic location. Klk6-induced loss of β-catenin in cell surface membranes was significantly reduced in astrocytes lacking PAR1−/− or PAR2−/−. β-catenin was visualized by immunofluorescence using an Alexa Fluor 488-conjugated secondary antibody along with Cy3-conjugated Phalloidin (see Figure 2) and DAPI stained nuclei (see Figure 4). (*P < 0.05, **P < 0.01, *** P < 0.001, NK). Scale bar = 50 μm.
Klk6-PAR astrocyte aggregation
Adoption of a stellate shape by astrocytes in response to Klk6 was accompanied by multicellular aggregation, with 1.8- to 2.5-fold increases compared to vehicle treatment alone (Figure 4). To determine the extent to which Klk6-evoked multicellular aggregation may be linked to activation of PAR, we quantified the distance between nuclei in Klk6 treated PAR+/+ cultures or those genetically lacking PAR1 or PAR2. Consistent with a key role for both PAR1 and PAR2 in Klk6-induced astrocyte aggregation, the absence of PAR1 or PAR2 significantly reduced or blocked the ability of Klk6 to promote aggregation. As we reported previously, Klk6 did not promote an increase in the number of DAPI stained nuclei indicative of proliferation, at least not under the conditions of this study during the 24 h treatment period examined (Scarisbrick et al. 2012b). These findings suggest that Klk6 promotes astrocyte aggregation by a mechanism that depends on activation of either PAR1 or PAR2.
Discussion
The present studies were undertaken to determine the contributions of PARs to Klk6-evoked changes in astrocyte form and function, with a focus on PAR1 and PAR2 (Vandell et al. 2008; Scarisbrick et al. 2012a; Radulovic et al. 2015; Radulovic et al. 2016). Our findings demonstrate that PAR1 plays an essential role in Klk6-evoked increases in intracellular Ca2+ in primary murine cortical astrocytes. We further demonstrate that both PAR1 and PAR2 play significant roles in Klk6-mediated astrocyte stellation, β-catenin translocation from the cell surface to the cytoplasm, and in astrocyte multicellular aggregation. Collectively the findings presented mechanistically connect changes in astrocyte dynamics that we have documented upon treatment with Klk6 (Vandell et al. 2008; Scarisbrick et al. 2012b; Radulovic et al. 2015) to both PAR1 and PAR2. Therefore, Klk6 and PARs are physiological partners regulating astrocyte form and function in the CNS and additional studies to determine how this signaling axis changes with injury and how it can be modulated to foster CNS function and repair will be important aspects of future investigations.
Klk6-PAR signaling regulates astrocyte intracellular Ca2+ levels
Proteinases are emerging as important regulators of cell behavior by receptor dependent and independent mechanisms. The current studies utilized murine cortical astrocytes with genetic PAR1-loss of function to definitively demonstrate that Klk6 elicits increases in astrocyte intracellular Ca2+ in a PAR1-dependent manner. These findings are supported by prior studies showing that a PAR1 small molecule inhibitor (SCH79797) effectively attenuates Klk6-evoked increases in astrocyte intracellular Ca2+ (Scarisbrick et al. 2012b). The current results also extend prior reports that Klk6 evokes increases in intracellular Ca2+ in the Neu7 astrocyte cell line by activating of PAR1 or PAR2 (Vandell et al. 2008). In primary murine cortical astrocytes however, the role of PAR2 was not prominent since PAR1-AP, but not PAR2-AP, significantly desensitized its cognate receptor to subsequent Klk6-mediated increases in Ca2+. These findings may reflect the more than 10-fold higher levels of PAR1 compared to PAR2 expression in primary astrocytes (Vandell et al. 2008), such that Klk6 continues to signal at PAR1, even after PAR2 receptor desensitization. We note that Neu7 astrocytes express at least 10-fold higher levels of PAR2 relative to primary astrocytes at rest and this may account for the ability of PAR2-AP to significantly desensitize subsequent Klk6-evoked Ca2+ signaling in this cell line, but not in primary astrocytes. It is also worth pointing out that although the Neu7 astrocyte cell line expresses significant levels of PAR4 RNA, that a PAR4-AP did not elicit a significant Ca2+ signal (Vandell et al. 2008). Oikonomopoulou et al., 2006a, showed that KLK6 was not able to activate rat platelet calcium signaling through PAR4. These studies do not rule out the possibility that Klk6 can activate PAR4 to drive non-Ca2+-dependent signaling and additional studies are needed in this regard. Since PAR3 is also expressed by astrocytes, can serve as a co-factor for PAR4 (Nakanishi-Matsui et al. 2000) and may signal on its own (Ostrowska et al. 2008), its role(s) in astrocyte biology will also be an important avenue of future investigation.
Differences in Klk6-activation of PAR underscore how the dynamics of Klk-PAR signaling may be modulated as the abundance of PARs and their agonists change in CNS injury and disease. With regard to astrocytes, PAR2 expression increases on astrocytes at sites of inflammation in human multiple sclerosis and in murine experimental autoimmune encephalomyelitis (Noorbakhsh et al. 2006). PAR1 also increases on astrocytes in human immunodeficiency virus encephalitis and in multiple sclerosis (Boven et al. 2003). This study also showed that activation of human astrocyte cultures with PAR1-AP increases expression of IL-1β and inducible nitric oxide synthase and the secretion of neurotoxic factors. Both PAR1 and PAR2 are also increased on astrocytes in the aftermath of SCI (Citron et al. 2000; Radulovic et al. 2015; Radulovic et al. 2016), in cortical trauma (Nicole et al. 2005), and ischemia (Rohatgi et al. 2004). Taken with dynamic changes in Klk6 in parallel neurological conditions (Scarisbrick et al. 1997; Scarisbrick et al. 2002; Terayama et al. 2004; Uchida et al. 2004; Scarisbrick et al. 2006; Scarisbrick et al. 2012c; Panos et al. 2014; Radulovic et al. 2015), the Klk6-PAR axis is well positioned to dynamically regulate astrocyte function in the intact and injured CNS.
The functional significance of Klk6-PAR-evoked elevations in intracellular Ca2+ in astrocytes warrants further study. While astrocytes do not generate action potentials, changes in intracellular Ca2+ enable astrocytes to exert their activities in a dynamic manner, including interactions with other astrocytes and glia, with synapses, and in the regulation of blood flow (Verkhratsky et al. 1998; Iadecola et al. 2007; Halassa et al. 2010; Tong et al. 2013). Of interest, prior studies show PAR-1 activation elicits astrocyte glutamate release, as detected by NMDA receptor-mediated slow inward currents in nearby neurons (Shigetomi et al. 2008).
Klk6-PAR signaling regulates astrocyte morphology
Genetic targeting of PAR1 or PAR2 significantly reduced Klk6-driven changes in astrocyte stellation, aggregation and translocation of β-catenin from the cell surface to the cytoplasm. We previously reported that Klk6 promotes astrocyte stellation in vitro in as little as 4 h and that this depends on enzymatic activity (Scarisbrick et al. 2012a). Also, a PAR1 small molecule inhibitor (SCH79797) attenuates Klk6-stellation. Here we used the power of genetics to definitively establish the role of PAR1 in mediating Klk6-astrocyte stellation in primary astrocytes. Findings here demonstrate for the first time that PAR2 is also a significant regulator of Klk6-mediated astrocyte stellation, since primary astrocytes lacking PAR2 showed marked reductions in Klk6-driven stellation. While the PAR gated signaling cascade(s) mediating Klk6-PAR-stellation remain to be fully elucidated, it is likely that protein kinase C (PKC) plays a prominent role, since the PKC inhibitor Go6983 blocks Klk6-astrocyte stellation (Scarisbrick et al. 2012a). We note that each of these studies examined astrocytes derived from cortices of the postnatal mouse brain and any differences across brain regions (Farmer et al. 2017; Yoon et al. 2017), or in aged astrocytes, will be an important line of future investigation.
Astrocyte stellation in response to Klk6 was accompanied by multicellular aggregation, an effect that was also highly dependent on the presence of PAR1 or PAR2. Astrocyte aggregation occurs in vivo in the aftermath of CNS trauma and in certain conditions where astrocytes band together to wall off a site of neuropathology from invading inflammatory cells and toxic cytokines (Hamby et al. 2012). The fact that astrocytes lacking PAR1 or PAR2 show less stellation and aggregation in cell culture in response to Klk6, supports prior in vivo findings that mice with global PAR1 or PAR2 gene knockout show significant reductions in hallmarks of astrogliosis after SCI, including reductions in expression of glial fibrillary acidic protein, vimentin and STAT3 signaling (Radulovic et al. 2015; Radulovic et al. 2016). The current findings support the idea that at least part of the in vivo effects of PAR loss-of-function after spinal cord trauma occur directly at the level of the astrocyte. Since mice lacking PAR1 or PAR2 show significant enhancements in overall recovery of function after SCI, the current findings lend support to the concept that PARs may serve as targets to modulate CNS glial scar formation in a manner that supports recovery of function.
The ability of Klk6 to promote multicellular aggregation is consistent with findings demonstrating that human kallikrein-related peptidase 7 (KLK7) promotes multicellular aggregation of ovarian cancer cells (Dong et al. 2010). In this case, KLK7-mediated aggregation was linked to up regulation of the alpha(5)beta(1) integrin pathway and chemoresistance. Interestingly, human kallikrein-related peptidase 4 (KLK4) likewise promotes ovarian cancer cell aggregation, however through a urokinase plasminogen activator-dependent mechanism (Dong et al. 2013). Human kallikrein-related peptidase 14 (Klk14), but not KLK5 or KLK6, promoted aggregation of rat platelets in a PAR4-dependent manner (Oikonomopoulou et al. 2006a; Oikonomopoulou et al. 2006b). Thus, it appears that multiple kallikrein-related peptidases are able to promote multicellular aggregation, albeit in a cell specific manner and through only partially overlapping mechanisms. Results presented here clarify that Klk6 promotes astrocyte aggregation by a mechanism that depends on intact PAR1 or PAR2.
Klk6-PAR mediated astrocyte stellation was associated with the movement of β-catenin from the cell surface to the cytoplasm. Cytoplasmic sequestration of β-catenin with subsequent translocation to the nucleus is linked to an epithelial-to-mesenchymal transition in multiple types of cancer (Kalluri et al. 2009). In the A549 tumor cell line, PAR2-activation by trypsin or matriptase increases expression of COX-2 in a β-catenin dependent manner (Wang et al. 2008). The possible regulation of β-catenin by KLK6 appears complex, since inhibition in a head and neck tumor cell line (FaDu) promotes an epithelial-to-mesenchymal transition, nuclear β-catenin accumulation and resistance to irradiation (Schrader et al. 2015). By contrast, KLK6 promotes resistance to irradiation and temozolomide in astrocytoma cell lines in a PAR1-dependent manner (Drucker et al. 2013; Drucker et al. 2015), with the role of β-catenin yet to be determined. In high-grade astrocytoma, nuclear β-catenin is associated with the invasive front of the tumor (Paul et al. 2013) and high levels of WNT/β-catenin are associated with poor patient survival (Sandberg et al. 2013). Taken with prior studies, the current findings point to Klk6-PAR as an important regulatory node for β-catenin signaling and additional efforts are needed to clarify the full impact on astrocyte biology in general and astrocytoma pathology in particular.
Conclusions
The ability of Klk6 to activate PAR and modulate astrocyte biology, including Ca2+ flux, stellation, β-catenin translocation and multicellular aggregation, in addition to IL-6 secretion, supports a model in which Klk6-PAR signaling participates in feed-forward and feed-back signaling cascades to promote astrogliosis (Radulovic et al. 2015; Radulovic et al. 2016). We therefore propose that Klk6 and/or the receptors it activates are ideally positioned as targets to modify astroglial physiology and the impact of astroglial dynamics on neural function in the intact, injured and regenerating CNS. In such a model we will need to consider that the nature of astrogliosis varies regionally in the CNS and with the type and state of the injury (Anderson et al. 2014; Morel et al. 2017; Yoon et al. 2017). Also, different inflammatory mediators generate markedly different GPCR and G protein expression and astrocyte calcium signaling transients (Hamby et al. 2012), including PAR expression (Noorbakhsh et al. 2005; Radulovic et al. 2015; Radulovic et al. 2016). These changes have the potential to alter astrocyte reactive properties in unique ways that may serve to counter stress responses, delimit the lesion and restore homeostasis acutely. In contrast, excessive astrogliosis may impair restoration of the vascular supply and synaptic connections thereby impairing recovery of function (Silver et al. 2004; Yuan et al. 2013). Therefore, additional information regarding the short and long term consequences of Klk6-PAR signaling in the continuum of astrogliosis will be needed to harness the potential power of this novel and disease relevant signaling axis to improve neurological outcomes.
Materials and Methods
Mice
The regulatory roles of PAR1 and PAR2 in mediating astroglial Ca2+ signaling and morphological changes in response to recombinant Klk6 was examined in primary astrocyte cultures derived from wild type mice or those with PAR1 or PAR2 gene knockout. Mice lacking PAR1 (PAR1−/−, B6.129S4-F2rtm1Ajc/J, Stock No. 002862), or PAR2 (PAR2−/−, B6.Cg-F2rl1tm1Mslb/J (former B6.Cg-F2rl1tm1Nwb/J, Stock No. 004993 (https://www.jax.org/strain/004993)), were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice have been backcrossed to C57BL/6J (Stock No. 000664, https://www.jax.org/strain/000664) for more than 45 generations such that astrocytes derived from C57BL/6J mice served as wild type controls (Radulovic et al. 2015; Radulovic et al. 2016). All animal experiments were carried out with adherence to National Institutes of Health Guidelines for animal care and safety and were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Astroglial cultures
Primary astrocytes were purified from mixed glial cultures prepared from the cortices of postnatal day 1 to 3 mice as previously detailed (Scarisbrick et al. 2012b; Burda et al. 2013; Yoon et al. 2013). Mixed glial cultures were grown in DMEM, 2 mM Glutamax, 1 mM sodium pyruvate, 20 mM HEPES, and 10% heat inactivated fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). All cultures contained a mix of astrocytes derived from male and female mice. After 10 days in vitro, contaminating oligodendrocyte progenitor cells were removed by overnight shaking and microglia subsequently eliminated by sequential panning on non-tissue culture treated plastic. Astrocytes purified under these conditions are greater than 95% pure based on immunoreactivity for ALDH1L1 (Radulovic et al. 2016).
Role of PAR in Klk6-mediated Ca2+ signaling
The ability of astrocyte PAR1 or PAR2 to gate Ca2+ signaling in response to Klk6 was determined using a series of receptor cross desensitization experiments involving recombinant Klk6 and peptides that specifically activate PAR1 (PAR1-AP, (TFLLR-amide) or PAR2 (PAR2-AP, SLIGRL-amide) without the need for proteolysis. All PAR-APs were purchased from Peptides International (Peptides International, Louisville KY). Recombinant Klk6 was expressed, purified and activated as previously described (Bernett et al. 2002; Blaber et al. 2002; Laxmikanthan et al. 2005; Scarisbrick et al. 2011; Scarisbrick et al. 2012b).
Purified cortical astrocytes from wild type mice were plated in serum containing media at a density of 21,400 cells/cm2 (15,000 cells/well) on Poly-L-lysine coated (10 μg/mL) glass chambered slides (Nalge Nunc International, Roskilde, Denmark) for 48 h. Prior to Ca2+ imaging, cells were loaded with the Rhod-3 calcium indicator (R10145, Life Technologies, Carlsbad, CA). In all experiments, changes in Rhod-3 fluorescence intensity after application of Klk6 or PAR-APs were performed in serum free media and captured with a 20X objective on an LSM 780 inverted confocal microscope (Carl Zeiss, Inc., Thornwood, NY). The excitation wavelength was 561 nm with emission collected at 595 nm. Rhod-3 loaded astrocytes were imaged for 2 min to establish the baseline fluorescence prior to application of Klk6 (150 nM (5 μg/ml)), PAR1-AP (40 μM), or PAR2-AP (100 μM) with changes in fluorescence monitored over the following 4 min. A receptor cross desensitization strategy was used to determine the extent to which changes in fluorescence observed after application of the first agonist were due to PAR1 or PAR2 activation. In these experiments, either PAR1 or PAR2-APs were applied 4 min after application of Klk6, with changes in fluorescence monitored for an additional 2 min. The converse experiments in which either PAR1- or PAR2-APs were applied prior to Klk6 were also examined. Reductions in fluorescence intensity observed when a second agonist is applied after the first had already activated PAR, is indicative of receptor desensitization and points to the ability of the first agonist to specifically activate PAR. Cells were selected across at least 3 wells per experiment for analysis, with a minimum of 15 cells being quantified in each segment of cross desensitization experiments. All results were verified using independent cell culture preparations. A baseline intensity value (F0) was created for each cell using the minimum intensity value collected. The intensity (F) of fluorescence emission responses were expressed as the mean ΔF/F0 = [(F – F0)/F0] and a line graph was created to illustrate data collected at one second intervals over the entire period of analysis. The mean peak changes in fluorescence ± s.e. were plotted as histograms in each case.
Role of PAR in Klk6-mediated astrocyte stellation
To test the hypothesis that PARs play an essential role in Klk6-mediated astrocyte stellation, we utilized a combination of recombinant kallikrein and primary cortical astrocytes derived from wild type mice or those genetically deficient in PAR1 or PAR2. Cortical astrocytes were plated across 12 mm poly-L-lysine (Sigma, St. Louis, MO) coated glass coverslips at a density of 15,000 cells per cm2 in serum containing media. After 24 h, media was replaced with defined Neurobasal A media containing 1% N2, 2% B27, 50 U/mL penicillin/streptomycin, 2 mM Glutamax, 1 mM sodium pyruvate, 0.45% glucose, and 50 μM β-mercaptoethanol (Sigma Aldrich, USA). All cells were maintained at 37°C in 95% air and 5% CO2. Cultures were treated in triplicate with recombinant Klk6 (30, 150 or 300 nM), or vehicle alone, for 24 h prior to fixation with 2% paraformaldehyde. Fixed astrocytes were subsequently permeabilized with 0.1% Triton X-100 and stained to visualize the actin cytoskeleton using Cy3-conjugated Phalloidin (1 U, Life Technologies). Alternatively, β-catenin was localized by immunofluorescence using a combination of a rabbit polyclonal primary antibody (ab16051, Abcam, Cambridge, MA) and an Alexa Fluor 488-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Labs, West Grove PA). All stained coverslips were slide mounted using Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei (Vector Laboratories, Burlingame CA). Five standardized 20X microscopic fields encompassing the poles and center of each coverslip were captured digitally on an Olympus AX70 microscope equipped with SPOT software (Olympus, Center Valley PA). Image J software was used to quantify the extent of astrocyte stellation, membrane or cytosplasmic localization of β-catenin, and nuclei aggregation (Vandell et al. 2008; Scarisbrick et al. 2012b).
Astrocyte stellation was defined as those cells possessing two or more processes at least one cell body in length and expressed as mean fold change compared to vehicle control in each case (Scarisbrick et al. 2012b). Astrocyte process length was measured using Interactive Pathology Laboratory software (BD Biosciences, Franklin Lakes NJ). Cell number in each case was determined by enumeration of DAPI stained nuclei in each microscopic field. The predominant localization β-catenin to the astrocyte plasma membrane or cytoplasm was scored based on immunofluorescence and expressed as fold change over vehicle alone. In each case, cells were treated in triplicate and experiments repeated at least twice. At least 300 cells were scored for each condition without knowledge of the experimental group.
Statistical analysis
The statistical significance of Klk6-elicited changes in astrocyte form and function across multiple groups was determined using One Way Analysis of Variance (ANOVA) with the Neuman Keuls (NK) post-hoc test, or non-parametric ANOVA on RANKS with Dunn’s test. Statistical differences between two groups were determined with Students t-test or Mann-Whitney U for non-parametric data. All data are presented as mean ± standard error of the mean (s.e.). In each case, P < 0.05 was considered statistically significant.
Acknowledgments
These studies were supported by R01NS052741 and RG3367 from the National Multiple Sclerosis Society to IAS. The authors gratefully acknowledge Dr. Michael Blaber for kindly providing the recombinant Klk6.
References
- Allen M, Ghosh S, Ahern GP, Villapol S, Maguire-Zeiss KA, Conant K. Protease induced plasticity: matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1. Sci Rep. 2016;6:35497. doi: 10.1038/srep35497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J Biol Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. [DOI] [PubMed] [Google Scholar]
- Anisowicz A, Sotiropoulou G, Stenman G, Mok SC, Sager R. A novel protease homolog differentially expressed in breast and ovarian cancer. Mol Med. 1996;2:624–636. [PMC free article] [PubMed] [Google Scholar]
- Ashby EL, Kehoe PG, Love S. Kallikrein-related peptidase 6 in Alzheimer’s disease and vascular dementia. Brain Res. 2010;1363:1–10. doi: 10.1016/j.brainres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. J Biol Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Scarisbrick IA, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- Borgono CA, Miacovos MP, Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- Boven LA, Vergnolle N, Henry SD, Silva C, Imai Y, Holden J, Warren K, Hollenberg MD, Power C. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J Immunol. 2003;170:2638–2646. doi: 10.4049/jimmunol.170.5.2638. [DOI] [PubMed] [Google Scholar]
- Burda JE, Radulovic M, Yoon H, Scarisbrick IA. Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia. 2013;61:1456–1470. doi: 10.1002/glia.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Smirnova IV, Arnold PM, Festoff BW. Upregulation of neurotoxic serine proteases, prothrombin, and protease- activated receptor 1 early after spinal cord injury. J Neurotrauma. 2000;17:1191–1203. doi: 10.1089/neu.2000.17.1191. [DOI] [PubMed] [Google Scholar]
- Diamandis EP, Yousef GM, Petraki C, Soosaipillai AR. Human kallikrein 6 as a biomarker of alzheimer’s disease. Clin Biochem. 2000;33:663–667. doi: 10.1016/s0009-9120(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Dong Y, Tan OL, Loessner D, Stephens C, Walpole C, Boyle GM, Parsons PG, Clements JA. Kallikrein-related peptidase 7 promotes multicellular aggregation via the alpha(5)beta(1) integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer research. 2010;70:2624–2633. doi: 10.1158/0008-5472.CAN-09-3415. [DOI] [PubMed] [Google Scholar]
- Dong Y, Stephens C, Walpole C, Swedberg JE, Boyle GM, Parsons PG, McGuckin MA, Harris JM, Clements JA. Paclitaxel resistance and multicellular spheroid formation are induced by kallikrein-related peptidase 4 in serous ovarian cancer cells in an ascites mimicking microenvironment. PloS one. 2013;8:e57056. doi: 10.1371/journal.pone.0057056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker KL, Paulsen AR, Giannini C, Decker PA, Blaber SI, Blaber M, Uhm JH, O’Neill BP, Jenkins RB, Scarisbrick IA. Clinical significance and novel mechanism of action of kallikrein 6 in glioblastoma. Neuro Oncol. 2013;15:305–318. doi: 10.1093/neuonc/nos313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker KL, Gianinni C, Decker PA, Diamandis EP, Scarisbrick IA. Prognostic significance of multiple kallikreins in high-grade astrocytoma. BMC Cancer. 2015;15:565. doi: 10.1186/s12885-015-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Murai K. Resolving Astrocyte Heterogeneity in the CNS. Front Cell Neurosci. 2017;11:300. doi: 10.3389/fncel.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Dal Maschio M, Beltramo R, Haydon PG, Benfenati F, Fellin T. Integrated brain circuits: neuron-astrocyte interaction in sleep-related rhythmogenesis. ScientificWorldJournal. 2010;10:1634–1645. doi: 10.1100/tsw.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, Nukina N. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 2003;100:13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Tokuda T, Yamaguchi N, Watanabe Y, Kametani F, Nakagawa M, Mizuno T. Cleavage of normal and pathological forms of alpha-synuclein by neurosin in vitro. Neurosci Lett. 2008;436:52–56. doi: 10.1016/j.neulet.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Laxmikanthan G, Blaber SI, Bernett MJ, Scarisbrick IA, Juliano MA, Blaber M. 1.70 A X-ray structure of human apo kallikrein 1: structural changes upon peptide inhibitor/substrate binding. Proteins. 2005;58:802–814. doi: 10.1002/prot.20368. [DOI] [PubMed] [Google Scholar]
- Little SP, Dixon EP, Norris F, Buckley W, Becker GW, Johnson M, Dobbins JR, Wyrick T, Miller JR, MacKellar W, Hepburn D, Corvalan J, McClure D, Liu X, Stephenson D, Clemens J, Johnstone EM. Zyme, a novel and potentially amyloidogenic enzyme cDNA isolated from Alzheimer’s disease brain. J Biol Chem. 1997;272:25135–25142. doi: 10.1074/jbc.272.40.25135. [DOI] [PubMed] [Google Scholar]
- Magklara A, Mellati AA, Wasney GA, Little SP, Sotiropoulou G, Becker GW, Diamandis EP. Characterization of the enzymatic activity of human kallikrein 6: Autoactivation, substrate specificity, and regulation by inhibitors. Biochem Biophys Res Commun. 2003;307:948–955. doi: 10.1016/s0006-291x(03)01271-3. [DOI] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, Yang Y. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci. 2017;37:8706–8717. doi: 10.1523/JNEUROSCI.3956-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4319–4329. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, Hollenberg MD, Power C. Proteinase-activated receptors in the nervous system. Nat Rev Neurosci. 2003;4:981–990. doi: 10.1038/nrn1255. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, McArthur JC, Silva C, Vodjgani M, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Yamada T, Tsujioka Y, Taguchi J, Takahashi M, Tsuboi Y, Fujino Y, Nakajima M, Yamamoto T, Akatsu H, Mitsui S, Yamaguchi N. Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer’s disease and Parkinson’s disease. Psychiatry Clin Neurosci. 2000;54:419–426. doi: 10.1046/j.1440-1819.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, Diamandis EP, Hollenberg MD. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006a;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- Oikonomopoulou K, Hansen KK, Saifeddine M, Vergnolle N, Tea I, Blaber M, Blaber SI, Scarisbrick I, Diamandis EP, Hollenberg MD. Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs) Biol Chem. 2006b;387:817–824. doi: 10.1515/BC.2006.104. [DOI] [PubMed] [Google Scholar]
- Ostrowska E, Reiser G. The protease-activated receptor-3 (PAR-3) can signal autonomously to induce interleukin-8 release. Cell Mol Life Sci. 2008;65:970–981. doi: 10.1007/s00018-008-7555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampalakis G, Sykioti VS, Ximerakis M, Stefanakou-Kalakou I, Melki R, Vekrellis K, Sotiropoulou G. KLK6 proteolysis is implicated in the turnover and uptake of extracellular alpha-synuclein species. Oncotarget. 2017;8:14502–14515. doi: 10.18632/oncotarget.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos M, Christophi GP, Rodriguez M, Scarisbrick IA. Differential expression of multiple kallikreins in a viral model of multiple sclerosis points to unique roles in the innate and adaptive immune response. Biol Chem. 2014;395:1063–1073. doi: 10.1515/hsz-2014-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul I, Bhattacharya S, Chatterjee A, Ghosh MK. Current Understanding on EGFR and Wnt/beta-Catenin Signaling in Glioma and Their Possible Crosstalk. Genes Cancer. 2013;4:427–446. doi: 10.1177/1947601913503341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- Prassas I, Eissa A, Poda G, Diamandis EP. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat Rev Drug Discov. 2015;14:183–202. doi: 10.1038/nrd4534. [DOI] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Larson N, Wu J, Linbo R, Burda JE, Diamandis EP, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein cascades in traumatic spinal cord injury: in vitro evidence for roles in axonopathy and neuron degeneration. Journal of neuropathology and experimental neurology. 2013;72:1072–1089. doi: 10.1097/NEN.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Wu J, Mustafa K, Fehlings MG, Scarisbrick IA. Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury. Neurobiol Dis. 2015;83:75–89. doi: 10.1016/j.nbd.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Wu J, Mustafa K, Scarisbrick IA. Targeting the thrombin receptor modulates inflammation and astrogliosis to improve recovery after spinal cord injury. Neurobiol Dis. 2016;93:226–242. doi: 10.1016/j.nbd.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput PS, Lyden PD, Chen B, Lamb JA, Pereira B, Lamb A, Zhao L, Lei IF, Bai J. Protease activated receptor-1 mediates cytotoxicity during ischemia using in vivo and in vitro models. Neuroscience. 2014;281C:229–240. doi: 10.1016/j.neuroscience.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Eissa A, Mihara K, Oikonomopoulou K, Saifeddine M, Renaux B, Diamandis E, Hollenberg MD. Proteinase-activated receptors (PARs): differential signalling by kallikrein-related peptidases KLK8 and KLK14. Biol Chem. 2012;393:421–427. doi: 10.1515/hsz-2011-0251. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Henrich-Noack P, Sedehizade F, Goertler M, Wallesch CW, Reymann KG, Reiser G. Transient focal ischemia in rat brain differentially regulates mRNA expression of protease-activated receptors 1 to 4. J Neurosci Res. 2004;75:273–279. doi: 10.1002/jnr.10847. [DOI] [PubMed] [Google Scholar]
- Sandberg CJ, Altschuler G, Jeong J, Stromme KK, Stangeland B, Murrell W, Grasmo-Wendler UH, Myklebost O, Helseth E, Vik-Mo EO, Hide W, Langmoen IA. Comparison of glioma stem cells to neural stem cells from the adult human brain identifies dysregulated Wnt- signaling and a fingerprint associated with clinical outcome. Exp Cell Res. 2013;319:2230–2243. doi: 10.1016/j.yexcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Towner MD, Isackson PJ. Induction of Serine Proteases in the Adult Rat Spinal Cord Following Kainic Acid Administration. Soc Neurosci Abs. 1996;22:746. [Google Scholar]
- Scarisbrick IA, Towner MD, Isackson PJ. Nervous system specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Asakura K, Blaber S, Blaber M, Isackson PJ, Beito T, Rodriguez M, Windebank AJ. Preferential expression of myelencephalon specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000;30:219–230. doi: 10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Lucchinetti CF, Genain CP, Blaber M, Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Sabharwal P, Cruz H, Larsen N, Vandell A, Blaber SI, Ameenuddin S, Papke LM, Fehlings MG, Reeves RK, Blaber M, Windebank AJ, Rodriguez M. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur J Neuroscience. 2006;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Epstein B, Cloud BA, Yoon H, Wu J, Renner DN, Blaber SI, Blaber M, Vandell AG, Bryson AL. Functional role of kallikrein 6 in regulating immune cell survival. PLoS One. 2011;6:e18376, 18371–18311. doi: 10.1371/journal.pone.0018376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA. Kallikrein Activity in the Central Nervous System. In: Schmitt M, Sommerhoff C, Fritz H, Magdolen V, editors. The Kallikreins. Berlin: De Gruyter Publishing; 2012. pp. 349–372. [Google Scholar]
- Scarisbrick IA, Blaber M. Kallikrein-related peptidase 6. In: Barrett AJ, Rawlings ND, editors. Handbook of Proteolytic Enzymes. London, UK: Elsevier; 2012a. pp. 2780–2786. [Google Scholar]
- Scarisbrick IA, Radulovic M, Burda JE, Larson N, Blaber SI, Giannini C, Blaber M, Vandell AG. Kallikrein 6 is a novel molecular trigger of reactive astrogliosis. Biological Chemistry. 2012b;393:355–367. doi: 10.1515/hsz-2011-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Yoon H, Panos M, Larson N, Blaber SI, Blaber M, Rodriguez M. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain Pathol. 2012c;22:709–722. doi: 10.1111/j.1750-3639.2012.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader CH, Kolb M, Zaoui K, Flechtenmacher C, Grabe N, Weber KJ, Hielscher T, Plinkert PK, Hess J. Kallikrein-related peptidase 6 regulates epithelial-to-mesenchymal transition and serves as prognostic biomarker for head and neck squamous cell carcinoma patients. Mol Cancer. 2015;14:107. doi: 10.1186/s12943-015-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Pampalakis G. Kallikrein-related peptidases: bridges between immune functions and extracellular matrix degradation. Biol Chem. 2010;391:321–331. doi: 10.1515/BC.2010.036. [DOI] [PubMed] [Google Scholar]
- Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:31–41. doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Valera E, Rockenstein E, Trejo-Morales M, Adame A, Masliah E. A brain-targeted, modified neurosin (kallikrein-6) reduces alpha-synuclein accumulation in a mouse model of multiple system atrophy. Mol Neurodegener. 2015;10:48. doi: 10.1186/s13024-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H, Watanabe Y, Kasai T, Mizuno T, Nakagawa M, Tanaka M, Tokuda T. Extracellular neurosin degrades alpha-synuclein in cultured cells. Neurosci Res. 2010;67:341–346. doi: 10.1016/j.neures.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Terayama R, Bando Y, Takahashi T, Yoshida S. Differential expression of neuropsin and protease M/neurosin in oligodendrocytes after injury to the spinal cord. Glia. 2004;48:91–101. doi: 10.1002/glia.20058. [DOI] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist. 2013;19:274–291. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- Uchida A, Oka Y, Aoyama M, Suzuki S, Yokoi T, Katano H, Mase M, Tada T, Asai K, Yamada K. Expression of myelencephalon-specific protease in transient middle cerebral artery occlusion model of rat brain. Brain Res Mol Brain Res. 2004;126:129–136. doi: 10.1016/j.molbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vandell AG, Larson N, Laxmikanthan G, Panos M, Blaber SI, Blaber M, Scarisbrick IA. Protease Activated Receptor Dependent and Independent Signaling by Kallikreins 1 and 6 in CNS Neuron and Astroglial Cell Lines. J Neurochem. 2008;107:855–870. doi: 10.1111/j.1471-4159.2008.05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- Wang H, Wen S, Bunnett NW, Leduc R, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through beta-catenin and cyclic AMP-response element-binding protein. J Biol Chem. 2008;283:809–815. doi: 10.1074/jbc.M703021200. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Tsuruoka N, Kodama S, Tsujimoto M, Yamamura Y, Tanaka T, Nakazato H, Yamaguchi N. Molecular cloning of a novel trypsin-like serine protease (neurosin) preferentially expressed in brain. Biochim Biophys Acta. 1997;1350:11–14. doi: 10.1016/s0167-4781(96)00187-x. [DOI] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Wu J, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity. Journal of neurochemistry. 2013;127:283–298. doi: 10.1111/jnc.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Walters G, Paulsen AR, Scarisbrick IA. Astrocyte heterogeneity across the brain and spinal cord occurs developmentally, in adulthood and in response to demyelination. PLoS One. 2017;12:e0180697. doi: 10.1371/journal.pone.0180697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–435. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghooni M, Soosaipillai A, Grass L, Scorilas A, Mirazimi N, Diamandis EP. Decreased concentration of human kallikrein 6 in brain extracts of Alzheimer’s disease patients. Clin Biochem. 2002;35:225–231. doi: 10.1016/s0009-9120(02)00292-8. [DOI] [PubMed] [Google Scholar]