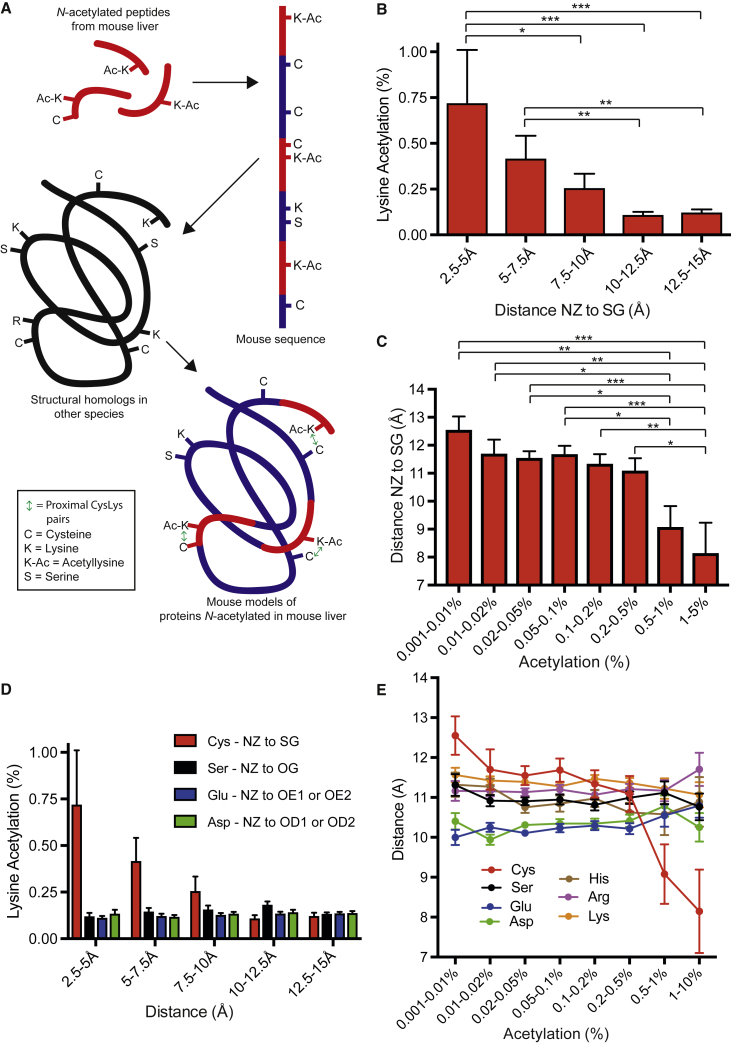
Figure 1.
Protein Lysine N-Acetylation Is Increased by Proximity to a Cysteine
(A) Creation of structural library of proteins with N-acetylated lysines in mouse liver in vivo. The set of 4,320 N-acetylated peptides is from Weinert et al. (2015). Mouse structural models of 619 proteins with N-acetylated lysines were generated based on molecular structures in other species, and the distance between their lysine amine (NZ) and cysteine thiol (SG) atoms was calculated.
(B) Lysine N-acetylation increases with proximity to a cysteine thiol. All N-acetylated CysLys pairs <15 Å apart were grouped by NZ to SG distance.
(C) The most N-acetylated lysines are closer to cysteine thiols. All N-acetylated CysLys pairs <15 Å apart were grouped by their degree of N-acetylation.
(D) Lysine N-acetylation is not caused by serines, glutamates, or aspartates. Pairs <15 Å apart were grouped by their NZ to SG, OG, OE1/OE2, or OD1/OD2 distances.
(E) The most N-acetylated lysines are not closer to serines, glutamates, aspartates, histidines, arginines, or other lysines. Pairs <15 Å apart were grouped by their degree of N-acetylation.
Data are the mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, not significant.