Figure 7.
Cell-Autonomous Suppression of DLL1 Function by NOTCH2NLB
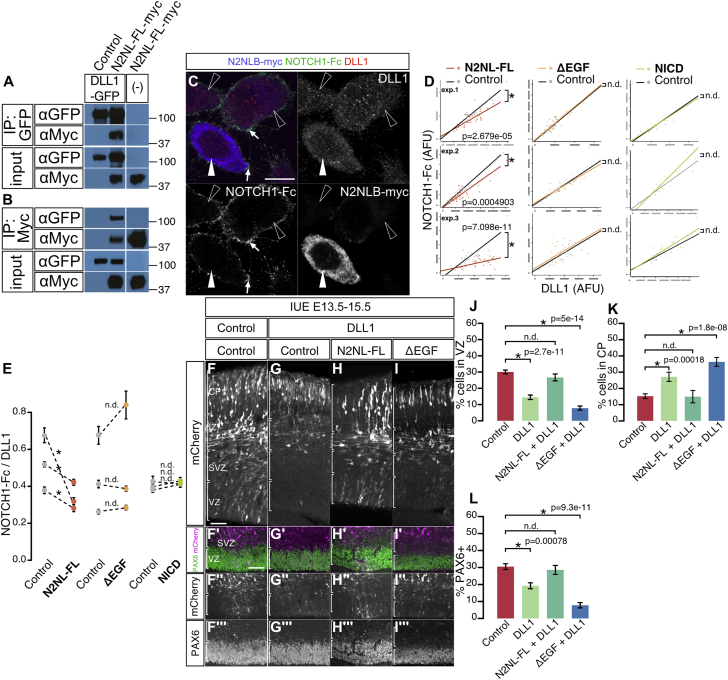
(A and B) Co-immunoprecipitation of NOTCH2NLB-myc (N2NL-FL-myc) and DLL1-GFP in HEK293T cells, using anti-GFP (A) and anti-Myc antibody (B).
(C) CHO cell line expressing DLL1 was transfected with a NOTCH2NLB expression plasmid. DLL1 protein at the plasma membrane was detected by NOTCH1-Fc (green in [C] and white arrows) and total DLL1 protein using DLL1 antibody (red in [C]). Anti-Myc antibody was used to identify NOTCH2NL-expressing cells (blue in [C], white arrowhead) among non-expressing cells (open arrowheads).
(D and E) The fluorescent intensities of NOTCH1-Fc (revealing DLL1 protein at the cell plasmic membrane) and of DLL1 antibody (revealing DLL1 protein in the whole cell) were measured in NOTCH2NLB-expressing cells and non-expressing cells (plots of three independent experiments). Compared to the non-expressing cells, NOTCH2NLB full-length-expressing cells show lower level of NOTCH-Fc signal, while NOTCH2NL-ΔEGF or NICD expressing cells display same levels as control cells (D). The ratio of signals for NOTCH1-Fc over DLL1 was quantified for each individual experiment (E).
(F–L) NOTCH2NLB suppresses DLL1 function in vivo. Mouse cortex in utero electroporation (E13.5, analysis at E15.5) was performed with DLL1 alone or together with N2NL-FL or N2NL-ΔEGF. Bin analysis reveals that the proportion of electroporated cells is decreased in VZ (J) and increased in CP (K) following DLL1 overexpression, which is blocked by NOTCH2NL-FL but not by NOTCH2NL-ΔEGF (F–K). The same results were obtained when examining the proportion of PAX6 progenitors in electroporated cells (F–I and L).
Data are presented as mean ± SEM and p values by Student’s t test (B and C) and one-way ANOVA and Bonferroni post-hoc test (H, I, and N). Scale bars, 10 μm (B) and 100 μm (E and K). See also Figure S5.