Abstract
Purpose
To report a fatal case of Susac syndrome in a 24-year-old female.
Observations
A 24-year-old female presented with progressive encephalopathy of unknown etiology. Her previous evaluation consisted of laboratory testing, imaging, and a brain biopsy to investigate for infectious and rheumatologic diseases. Several months after onset of symptoms, she underwent ophthalmic examination, which demonstrated bilateral branch retinal artery occlusions. Further review of her medical record revealed a recent history of hearing loss. Based on the retinal and systemic findings, the patient was diagnosed with Susac syndrome. The patient was started on intensive immunosuppression; however, she became more obtunded and succumbed several months after her diagnosis.
Conclusions and importance
The timely and accurate diagnosis of Susac syndrome, which classically manifests as the triad of encephalopathy, vestibulocochlear abnormalities, and retinal arteriolar occlusions, may help to reduce the morbidity of invasive testing and to prevent fatality
Keywords: Susac syndrome, Retinal artery occlusion, Encephalopathy
1. Introduction
Susac syndrome is a rare autoimmune endotheliopathy leading to microvascular occlusions in the brain, retina and inner ear.1 The syndrome classically manifests as the triad of encephalopathy, retinal artery occlusions, and vestibulocochlear dysfunction, although only 13% of patients display the complete triad at presentation.1 On average, there is a 21-week delay between symptom onset and completeness of the triad.1 The disease has a prevalence of 4.8 per million and primarily affects young, white females.2 Three distinct clinical courses have been described including monocyclic (fluctuating and self-limiting), polycyclic (relapsing and persistent longer than 2 years), and rarely, a chronic-progressive and potentially fatal course.1,3 We report a fulminant case of Susac syndrome which was undiagnosed until a dilated fundus examination revealed bilateral branch retinal artery occlusions (BRAO), completing the clinical triad of this disease.
2. Case report
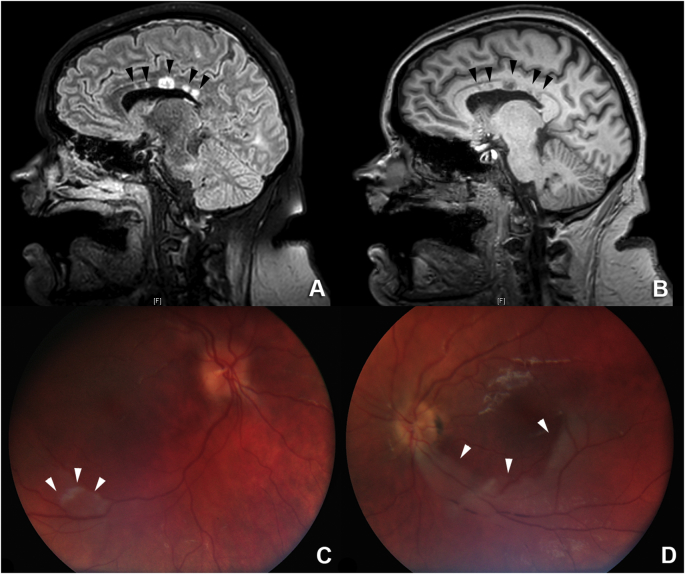
A 24-year old female presented to an outside hospital with a two-month history of worsening somnolence, confusion and new hallucinations. She had a history of ethanol, marijuana and tobacco abuse for many years. She became progressively encephalopathic and required intubation one month after her initial presentation. An extensive infectious and rheumatologic workup, including a dedicated cerebral spinal fluid autoimmune panel, were non-diagnostic. Magnetic resonance imaging (MRI) of the brain revealed T2 hyperintensities in the central corpus callosum with corresponding ‘punched-out-holes’ on sagittal T1. Additional T2 hyperintensities were present in the subcortical and deep white matter and numerous foci of restricted diffusion were present throughout both cerebral hemispheres (images not shown). The only pathologic examination included a non-diagnostic brain biopsy (Fig. 1A, B, and C) which demonstrated a chronic inflammatory meningoencephalitis. She received empiric treatment with acyclovir, high-dose steroids, intravenous immunoglobulin (IVIG) and plasma exchange (PLEX), with temporary neurologic improvement after steroid treatment. Once the patient was transferred to Stanford Hospital, repeat MRI of the brain was performed and demonstrated persistent white matter lesions (Fig. 2A and B). Approximately 3 months after the patient initially presented to the outside hospital, ophthalmology was consulted for a dilated fundus examination. Retinal evaluation at Stanford (SJS, SRS) revealed branch artery occlusions along the inferotemporal arcades in both eyes (Fig. 2C and D). Gass plaques and retinal artery collaterals were not appreciated on clinical examination. Additional review of the medical records revealed a recent episode of hearing loss and the development of migraines within the prior year. The hearing loss was not formally evaluated with audiology testing. Based on the constellation of findings, the patient was diagnosed with Susac syndrome and intravenous cyclophosphamide was initiated. However, despite the initiation of more aggressive therapy after the ophthalmic examination, the patient failed to demonstrate any improvement in mental status. The patient was transferred back to a hospital closer to home where she died several months later. An autopsy was not performed, and her cause of death has been attributed to neurologic compromise secondary to fulminant Susac Syndrome.
Fig. 1.
A: Low powered histopathologic specimen demonstrating lymphohistiocytic infiltration of the brain tissue. B: High powered histopathologic specimen of Fig. 1A. C: Histopathologic specimen showing perivascular inflammation.
Fig. 2.
A: Sagittal T2 hyperintensities (black arrows) in the central corpus callosum. B. Sagittal T1 “punched-out-holes” (black arrows) in the central corpus callosum that correspond to the T2 hyperintensities. C: Fundus photo of the right eye demonstrating retinal whitening (white arrows) adjacent to the inferotemporal arcade consistent with a BRAO. D: Fundus photo of the left eye demonstrating retinal whitening (white arrows) of the inferior macula with associated “box-carring” of the retinal vasculature consistent with a BRAO.
3. Discussion
The diagnosis of Susac syndrome relies on multimodal imaging and subspecialty evaluation. Encephalopathy and headaches are the most common presenting symptoms, followed by vision and then hearing abnormalities.1 The classic clinical triad of central nervous system, vision and hearing abnormalities is present in only 13% of patients on initial presentation, though 85% of patients will develop the complete triad during the course of their disease.1 The presence of white matter lesions particularly of the corpus callosum on MRI is nearly pathognomonic, and were present in 98% of reviewed cases.1,4 Of patients undergoing fluorescein angiography (FA), 99% demonstrated branch retinal artery occlusions, highlighting the diagnostic value of ophthalmic examination, FA and neuroimaging in suspected cases of Susac syndrome.1 In the present case, the patient was hospitalized and FA could not be performed which may have demonstrated arterial wall hyperfluorescence and arterial collaterals. However, the retinal examination clearly showed bilateral BRAOs and “box carring” of the retinal vasculature. Prior to the ophthalmic examination and diagnosis of Susac syndrome, the patient underwent a brain biopsy, which was non-diagnostic. Previously reported brain biopsy specimens have typically demonstrated lymphocytic infiltration with endothelial cell necrosis consistent with an inflammatory microangiopathy.5, 6, 7, 8, 9 The brain biopsy specimen in this case demonstrated features consistent with meningoencephalitis, including lymphohistiocytic infiltration, astrogliosis, and reactive microglia. There was also evidence of perivascular inflammation (Fig. 2C). Brain biopsy specimens are not necessary for the diagnosis of Susac syndrome and they are rarely obtained in suspected cases.5, 6, 7, 8, 9 The index case highlights the value of an ophthalmic examination in the evaluation for Susac syndrome and emphasizes the utility of a complete ophthalmic examination before undertaking more invasive procedures.
The initial treatment of patients with Susac syndrome, particularly patients with severe encephalopathy, is high-dose intravenous steroids and IVIG.10 More potent immunosuppressive agents, including cyclophosphamide, rituximab, and TNF-inhibitors have been utilized for refractory disease.10 Once remission is achieved, a slow steroid taper with sustained treatment with steroid sparing immunomodulation is typically required for an average of two years.1,10 The use of antithrombotics, including aspirin has been used, though there is no data to demonstrate its efficacy in Susac syndrome.11 In this case, the patient received high dose steroids, IVIG, and plasmapheresis prior to the diagnosis of Susac syndrome. Despite the aggressive therapy, there was only minimal improvement in her encephalopathy. Following the diagnosis of Susac syndrome, cyclophosphamide was initiated, although the patient remained encephalopathic and unfortunately succumbed several months later. While we cannot definitively rule out an idiopathic cause of death, progressive Susac syndrome was deemed the likely etiology.
Chronic-progressive cases of Susac syndrome requiring extensive immunomodulation are rare. To our knowledge, there are only two reported cases of Susac syndrome that potentially resulted in the death of the patient.3,12 Saux et al. described a 62 year old female who died 12 weeks after her diagnosis of Susac syndrome despite intravenous steroids, IVIG and cyclophosphamide.3 Hantson et al. described a fatal case of encephalopathy in 22 year old male with a history of marijuana and cocaine abuse.12 The patient was found to have corpus callosal lesions on MRI, bilateral BRAOs and died 3 months after admission despite high dose systemic corticosteroids. The authors implicated multifocal inflammatory leukencephalopathy associated with levamisole (an adulterant of cocaine) as the cause of death.12 They questioned the diagnosis of Susac syndrome because they were unable to confirm auditory dysfunction and believed the rapid progression to death over the course of months was extremely unusual for Susac syndrome. However, we believe the case by Hantson et al. likely represents another fatal case of Susac syndrome given the pathognomic MRI and retinal findings.4,12 It is not clear what distinguishes chronic-progressive Susac syndrome cases from the more typical monocyclic and polycyclic subtypes. It is possible that in some cases chronic marijuana abuse may accelerate the clinical course, leading to fatality. Of the three cases of fatal Susac syndrome (including this report), two were reported to be chronic marijuana abusers. Marijuana has been associated with peripheral vascular disease and cerebral vascular disease which in the setting of a vasculitis such as Susac syndrome may be the inciting event to a progressive, unrelenting, and potentially fatal outcome.13,14
4. Conclusions
Patients with encephalopathy of unknown etiology should undergo dilated fundus examination to evaluate for signs of arteriolar occlusion which, if found, may support the diagnosis of Susac syndrome or other underlying systemic disease. Early diagnosis minimizes the risk of invasive testing and may enable expedited initiation of immunosuppressive and/or other appropriate therapy for these patients.
Patient consent
The mother of the patient consented to the preparation of this case report.
Acknowledgements and disclosures
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: RS, RK, SS, SS, QDN.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
The Byers Eye Institute at Stanford University (P30-026877) is a recipient of the Research to Prevent Blindness Unrestricted Grant to support education and research.
References
- 1.Dörr Jan, Krautwald Sarah, Wildemann Brigitte. Friedemann Paul & Ilka Kleffner Characteristics of Susacc syndrome: a review of all reported cases. Nat Rev Neurol. June 2013;9:307–316. doi: 10.1038/nrneurol.2013.82. [DOI] [PubMed] [Google Scholar]
- 2.Seifert-Held T., Langner-Wegscheider B.J., Komposch M. Susacc's syndrome: clinical course and epidemiology in a Central European population. Int J Neurosci. 2016:1–5. doi: 10.1080/00207454.2016.1254631. (ISSN: 1563-5279) [DOI] [PubMed] [Google Scholar]
- 3.Saux A., Niango G., Charif M. Susacc's syndrome, a rare, potentially severe or lethal neurological disease. J Neurol Sci. 2010;297(1–2):71–73. doi: 10.1016/j.jns.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Susac J.O., Murtagh F.R., Egan R.A. MRI findings in Susacc's syndrome. Neurology. 2003;61(12):1783–1787. doi: 10.1212/01.wnl.0000103880.29693.48. [DOI] [PubMed] [Google Scholar]
- 5.Heiskala H., Somer H., Kovanen J., Poutiainen E., Karli H., Haltia M. Microangiopathy with encephalopathy, hearing loss and retinal arteriolar occlusions: two new cases. J Neurol Sci. 1988;86:239–250. doi: 10.1016/0022-510x(88)90102-5. [DOI] [PubMed] [Google Scholar]
- 6.O'Halloran H.S., Pearson P.A., Lee W.B., Susac J.O., Berger J.R. Microangiopathy of the brain, retina, and cochlea (Susac syndrome). A report of five cases and a review of the literature. Ophthalmology. 1998;105:1038–1044. doi: 10.1016/S0161-6420(98)96005-5. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro M.L.R., Swanson R.A., Coppeto J.A., Cuneo R.A., DeArmond S.J., Prusiner S.B. A microangiopathic syndrome of encephalopathy, hearing loss, and retinal arteriolar occlusions. Neurology. 1985;35:1113–1121. doi: 10.1212/wnl.35.8.1113. [DOI] [PubMed] [Google Scholar]
- 8.Kaminska E.A., Sadler M., Sangalang V., Hoskinmott A., Silverberg D. Microangiopathic syndrome of encephalopathy, retinal vessel occlusion, and hearing loss. Can J Neurol Sci. 1990;17:241. [Google Scholar]
- 9.Fox R.J., Costello F., Judkins A.R. Treatment of Susac syndrome with gamma globulin and corticosteroids. J Neurol Sci. 2006;251:17–22. doi: 10.1016/j.jns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Bitra R.K., Eggenberger E. Review of susac syndrome. Curr Opin Ophthalmol. 2011 Nov;22(6):472–476. doi: 10.1097/ICU.0b013e32834bbfeb. [DOI] [PubMed] [Google Scholar]
- 11.Vodopivec I., Prasad S. Treatment of susac syndrome. Curr Treat Options Neurol. 2016;18(1):3. doi: 10.1007/s11940-015-0386-x. [DOI] [PubMed] [Google Scholar]
- 12.Hantson P., Di fazio V., Del mar ramirez fernandez M., Samyn N., Duprez T., Van pesch V. Susac-like syndrome in a chronic cocaine abuser: could levamisole play a role? J Med Toxicol. 2015;11(1):124–128. doi: 10.1007/s13181-014-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desbois A.C., Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27(7):996–1005. doi: 10.1016/j.avsg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Santos R.P., Resende C.I., Vieira A.P., Brito C. Cannabis arteritis: ever more important to consider. BMJ Case Rep. 2017:2017. doi: 10.1136/bcr-2016-219111. [DOI] [PMC free article] [PubMed] [Google Scholar]