Figure EV1. Sgt2 prevents TA aggregation only at super‐physiological concentrations.
-
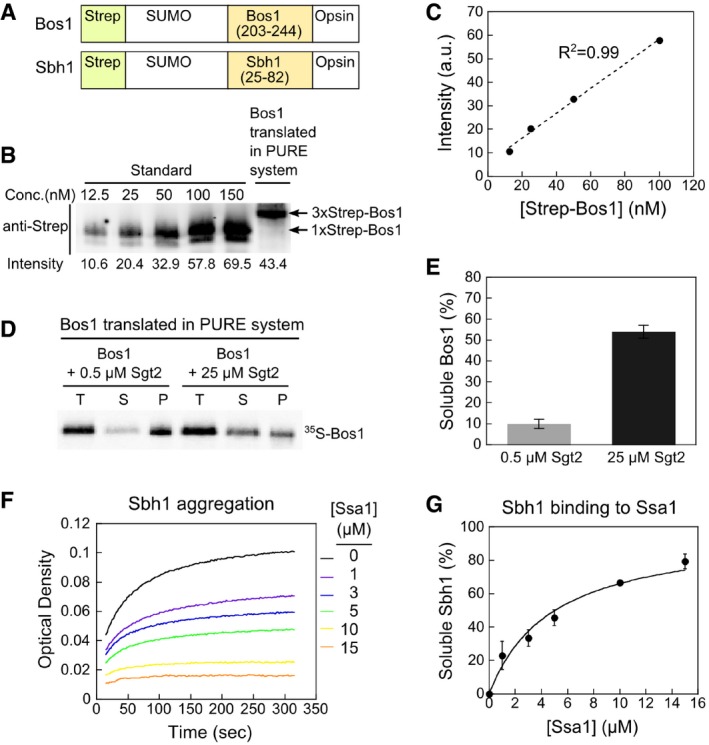
ASchematic of the model TA substrates used in in vitro assays. A Strep‐tagged non‐cleavable SUMO protein was fused to Bos1 residues 203–244 or Sbh1 residues 25–82 that encompasses their targeting sequence, which includes an N‐terminal linker to the cytosolic domain, the TMD, and the C‐terminal sequence element that regulates insertion into the ER (Rao et al, 2016). An opsin tag was attached to C‐terminus of model TAs. For IVT, a 3xStrep tag was fused to SUMO in Bos1 instead of 1xStrep tag.
-
B, CQuantitative Western blot analysis of Bos1 synthesized in the PURE‐IVT system. Known amounts of purified Strep‐Bos1 were used to construct a standard curve (panel B) for quantification of the concentration of 3xStrep‐Bos1 translated in the PURE system. Proteins were detected with a secondary antibody labeled with a near‐infrared fluorescent dye, IRDye. The 3xStrep‐Bos1 synthesized in PURE‐IVT was 71 nM, which is sub‐stoichiometric to the chaperones used in this work.
-
D, ESedimentation analysis of 35S‐labeled Bos1 synthesized by PURE‐IVT supplemented with 0.5 μM or 25 μM Sgt2. After ultracentrifugation, total input (T), soluble (S), and pellet (P) fractions were resolved by SDS–PAGE and visualized by autoradiography. The quantification of soluble Bos1 at indicated Sgt2 concentrations is shown in (E). All values are mean ± SD, with n ≥ 3.
-
F, GSsa1 suppresses Sbh1 aggregation in a dose‐dependent manner in the turbidity assay. Panel (G) shows the quantification of the data in (F) and replicates. The line was a fit of the data to equation (1) and gave a K soluble value of 4.8 ± 0.9 μM. All values are reported as mean ± SD, with n = 2.
