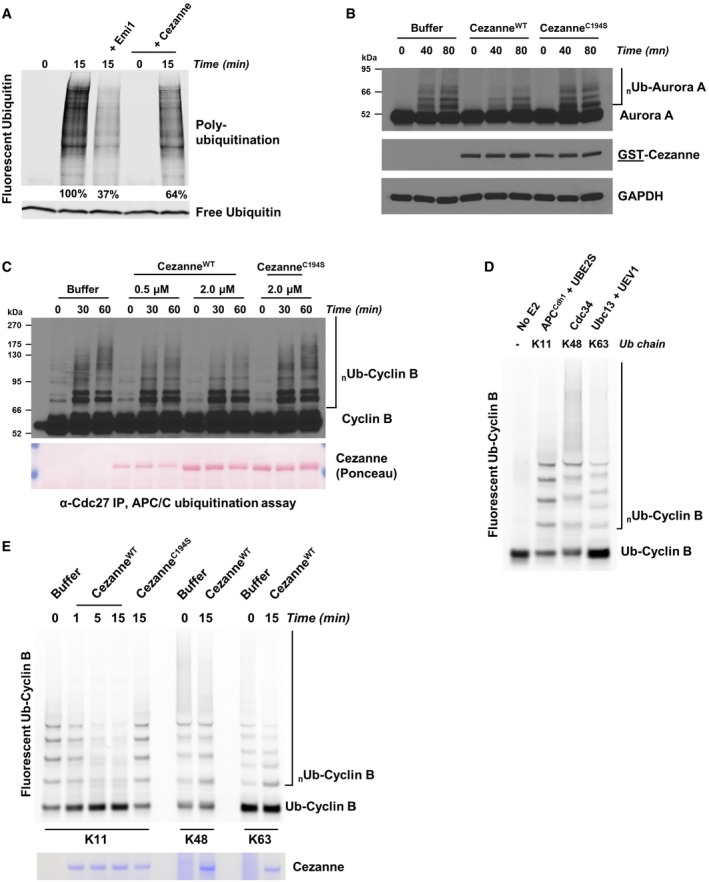
G1 extract from HeLaS3 cells was prepared and mixed with ATP, UBE2C, UBE2S, and fluorescent ubiquitin. Where indicated, recombinant Emi1 or Cezanne was also added. Reactions were incubated at 25°C and analyzed by SDS–PAGE and fluorescence scanning.
G1 extract from HeLaS3 cells was prepared and mixed with ATP, UBE2C, ubiquitin, and either 2 μM of recombinant CezanneWT or CezanneC194S. Reactions were incubated at room temperature and analyzed by SDS–PAGE and immunoblot.
APC/C was immunopurified from mitotic HeLaS3 cell extracts using anti‐Cdc27 beads and then mixed with in vitro‐translated Cyclin B, E1, E2, ATP, ubiquitin, and indicated amounts of Cezanne. Aliquots were collected at the indicated time points and analyzed by immunoblot using Cyclin B antibodies.
Ubiquitination reactions of a fluorescein‐labeled substrate N‐terminal fragment of Cyclin B fused to ubiquitin (Ub‐Cyclin B) were carried out in the presence of either recombinant APC/CCdh1, Cdc34, or Ubc13 and UEV1 to generate the indicated ubiquitin chain topologies. Reactions were quenched with EDTA and then analyzed by SDS–PAGE and fluorescence scanning.
Fluorescein‐labeled Ub‐Cyclin conjugated to K11, K48, or K63 ubiquitin chains was incubated with recombinant Cezanne for the indicated times and then analyzed by SDS–PAGE, fluorescence scanning, and Coomassie staining.