FIGURE 4.
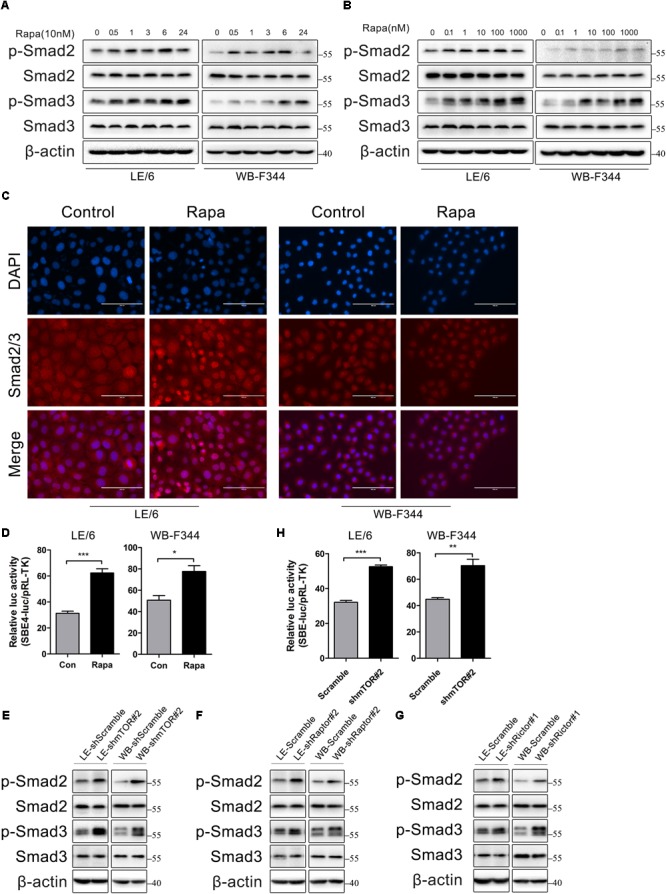
Rapamycin actives TGF-β-Smad signaling in HPCs. (A) LE/6 and WB-F344 cells were stimulated with rapamycin (10 nM) for the indicated times before cells were harvested for immunoblotting analysis against indicated proteins. β-actin was used as a loading control; (B) LE/6 and WB-F344 cells were treated with rapamycin at indicated concentrations for 6 h. Lysates were subjected to Western blot analysis with antibodies against indicated proteins. β-actin was used as a loading control; (C) LE/6 and WB-F344 cells were treated with rapamycin (10 nM) for 6 h and then subjected to immunofluorescent staining of Smad2/3 (red); DAPI were used to show the location of the nucleus (blue); scale bar, 100 μm; (D) LE/6 and WB-F344 cells were co-transfected with pRL-TK and CTGF-luc plasmids and then treated with Rapamycin for 16 h. Luciferase activity was normalized to renilla luciferase activity. Results showed as means ± SD. of triplicate measurements. ∗p < 0.05, ∗∗∗p < 0.001; (E–G) Lysates of LE-shmTOR#2, WB-shmTOR#2, LE-shRaptor#2, WB-shRaptor#2, LE-shRictor#1, WB-shRictor#1 and their scramble control (shScramble) were subjected to Western blot analysis with antibodies against indicated proteins. β-actin was used as a loading control; (H) LE-shmTOR#2, WB-shmTOR#2 and their scramble control (shScramble) were co-transfected with pRL-TK and SBE4-luc plasmids for 24 h. Luciferase activity was normalized to renilla luciferase activity. Results showed as means ± SD. of triplicate measurements. ∗∗p < 0.01, ∗∗∗p < 0.001.
