Table 2. Hydrogenation of benzanilide 2 to aniline 3 and benzyl alcohol 4 with manganese complex Mn-2: fine tuning of reaction conditions.
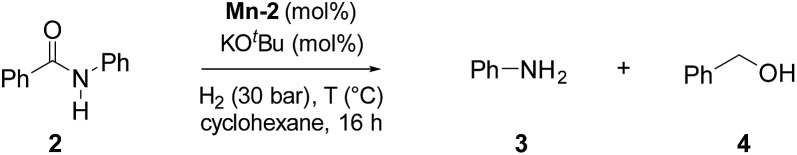
| ||||||
| Entry a | T (°C) | Mn-2 (mol%) | KOtBu (mol%) | Conv. b (%) | 3 b (%) | 4 b (%) |
| 1 | 120 | 1.5 | 8 | >99 | 96 | 90 |
| 2 | 100 | 2 | 10 | >99 | 96 | 90 |
| 3 | 100 | 2 | 8 | 95 | 88 | 80 |
| 4 | 100 | 2 | 5 | 29 | 24 | 24 |
| 5 c | 100 | 2 | 10 | 58 | 57 | 44 |
| 6 | 100 | 1.5 | 10 | 79 | 67 | 60 |
| 7 | 80 | 2 | 10 | 18 | 8 | — |
| 8 d | 100 | 2 | 10 | — | — | — |
| 9 e | 100 | 2 | 10 | — | — | — |
| 10 f | 100 | 2 | 10 | 63 | 53 | 50 |
aStandard reaction conditions: benzanilide 2 (49.30 mg, 0.25 mmol), Mn-2 (1.5–2 mol%), KOtBu (5–10 mol%), cyclohexane (2 mL), 15–30 bar of H2, 100–120 °C over 16 h.
bConversion of 2 and yields of 3 and 4 were calculated by GC using hexadecane as external standard.
cRun at 15 bar of H2.
dRun with 2 mol% of ligand 1 in the absence of Mn-2 complex.
eRun with 2 mol% of Mn(CO)5Br in the absence of Mn-2 complex.
fRun with 2 mol% of ligand 1 and 2 mol% of Mn(CO)5Br.
