Table 3. Substrate scope in the hydrogenation of amides to alcohols and amines catalyzed by Mn-2 complex.
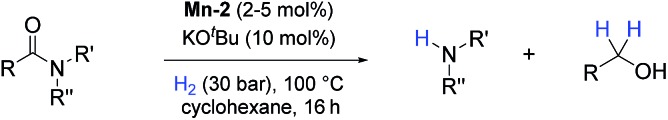
| |||||
| Entry a | Amide | Mn-2 (mol%) | Conv. b (%) | Yield of amine b (%) | Yield of alcohol b (%) |
| 1 |
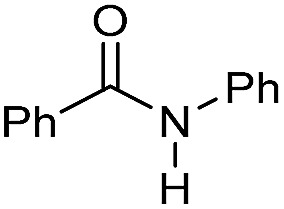
|
2 | >99 | 96 | 90 |
| 2 |
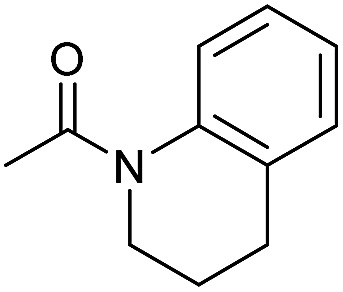
|
2 | 94 | 88 | 85 |
| 3 |
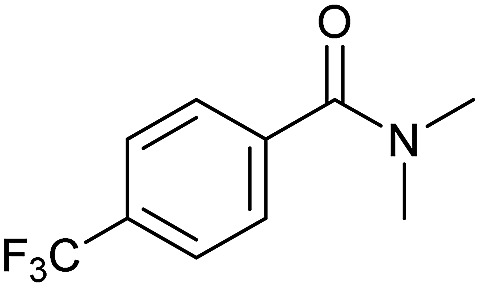
|
2 | >99 | 90 | n.d. |
| 4 |
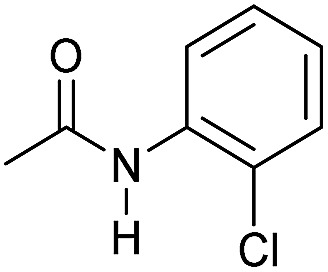
|
2 | 90 | 84 | 80 |
| 5 |
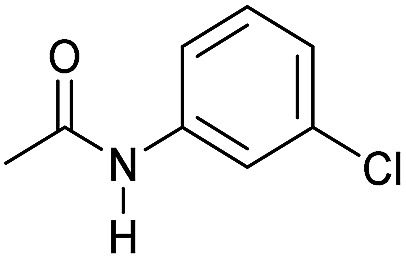
|
4 | 85 | 81 | 75 |
| 6 |
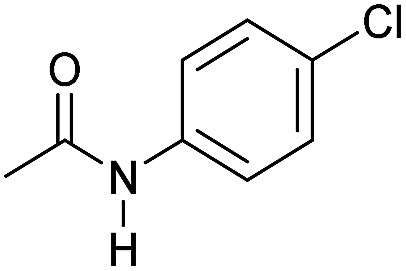
|
4 | >99 | >99 | >99 |
| 7 |
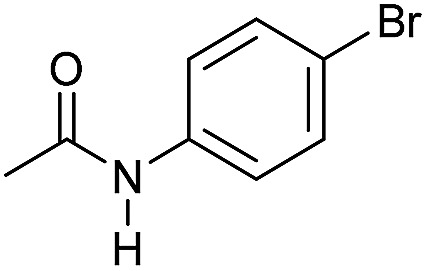
|
3 | 94 | 85 | 80 |
| 8 c |
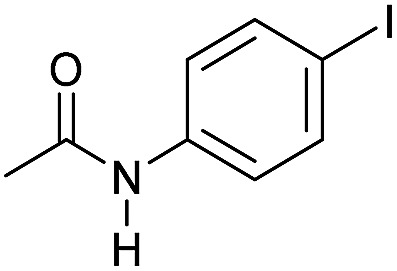
|
3 | >99 | >99 | 90 |
| 9 |
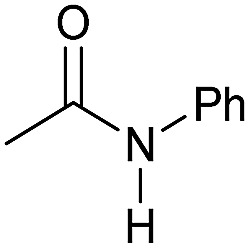
|
4 | 95 | 94 | 90 |
| 10 |
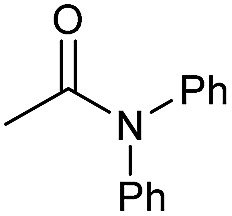
|
4 | 85 | 82 | 80 |
| 11 |
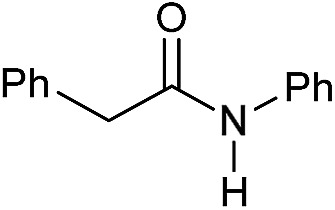
|
4 | >99 | 98 | 94 |
| 12 |
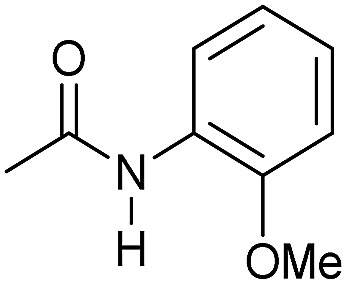
|
4 | 95 | 92 | 90 |
| 13 c |
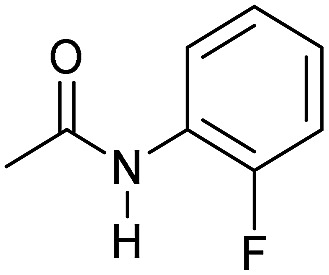
|
4 | >99 | >99 | >99 |
| 14 d |
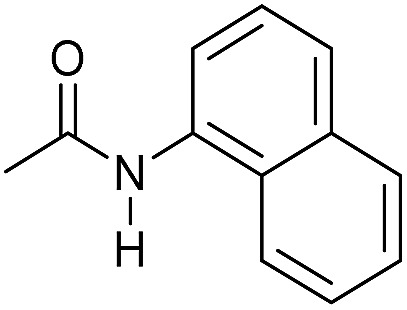
|
2 | 95 | 90 | 85 |
| 15 c , d |
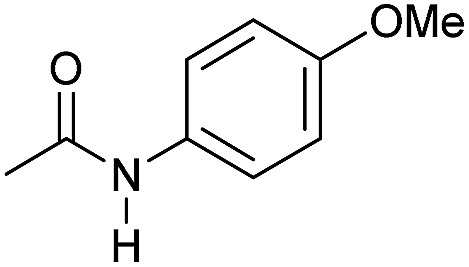
|
2 | 82 | 81 | 78 |
| 16 d |
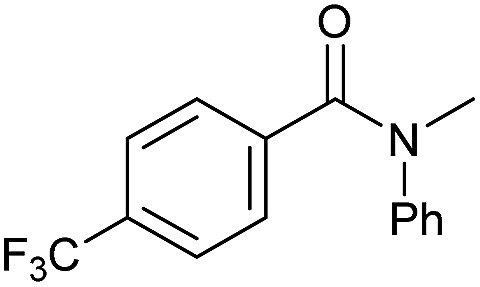
|
3 | >99 | >99 | >99 |
| 17 c , d |
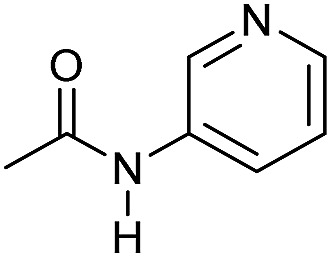
|
4 | >99 | >99 | 90 |
| 18 c , d |
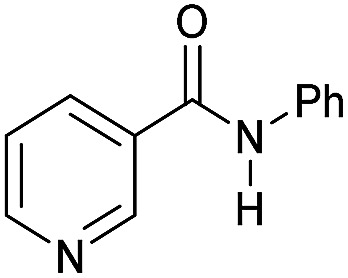
|
5 | 98 | 97 | 96 |
aStandard reaction conditions: amide (0.25 mmol), Mn-2 cat. (2–5 mol%), KOtBu (2.8 mg, 0.025 mmol, 10 mol%), cyclohexane (2 mL), 30 bar of H2, 100 °C over 16 h.
bConversion of the amide and yield of the alcohol and amine were calculated by GC using hexadecane as external standard.
cThe reaction was carried out using cyclohexane/t-amylOH (1.5/0.5) mixture as solvent (2 mL).
dRun at 120 °C.
