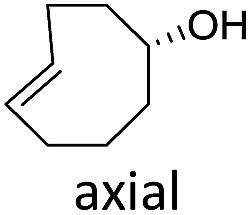
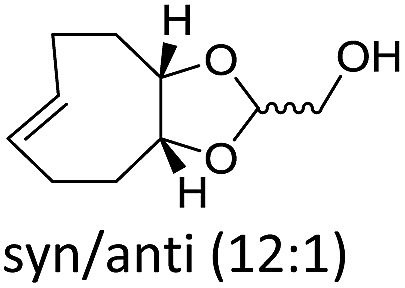
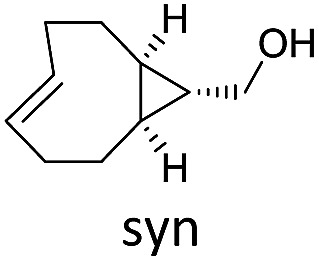
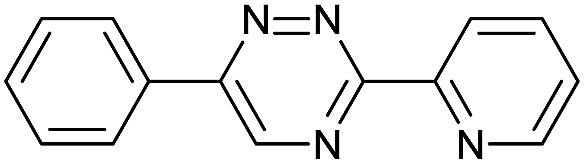
Table 1. Second-order rate constants (in M–1 s–1 × 10–2) of the reaction between 1,2,4-triazines and TCOs a .
| Triazine/TCO |
|
|
|
|
| 1 |
|
2.5 ± 0.1 | 56 ± 0.8 | 190 ± 3.0 |
| 2 |
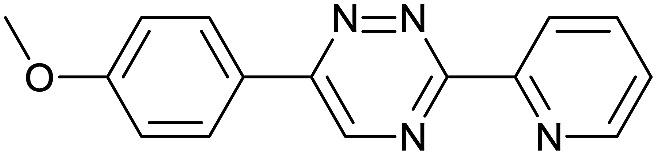
|
2.1 ± 0.2 | 36 ± 0.3 | 120 ± 1.0 |
| 3 |
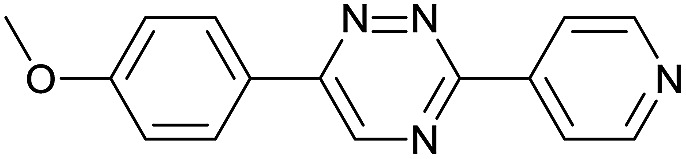
|
1.7 ± 0.4 | 35 ± 2.0 | 140 ± 3.0 |
| 4 |
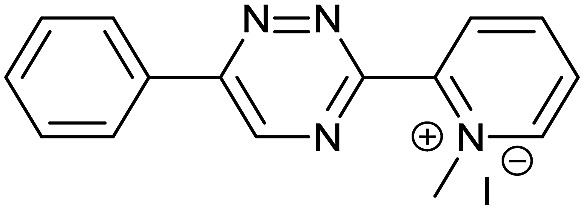
|
0.7 ± 0.1 | 8.3 ± 1.3 | 30 ± 1.0 |
| 5 |
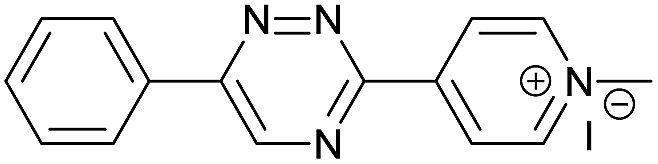
|
19.3 ± 0.2 | 260 ± 4.0 | 990 ± 30.0 |
| 6 |
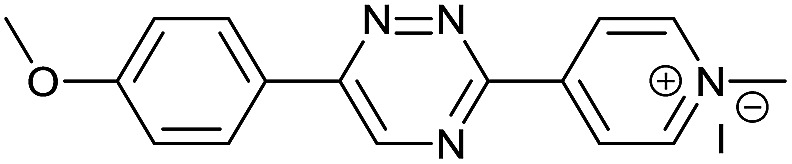
|
9.1 ± 0.3 | 190 ± 6.0 | 640 ± 20.0 |
| 7 |
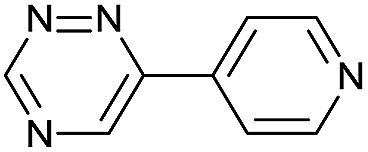
|
79.0 ± 5.0 | 940 ± 20.0 | 2020 ± 32.0 |
| 8 |
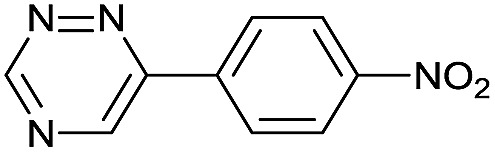
|
84.0 ± 7.0 | 1130 ± 50.0 | 2450 ± 70.0 |
aAll reactions were performed in H2O/CH3CN = 1/1 at room temperature under pseudo first-order conditions using an excess of the corresponding TCO.
