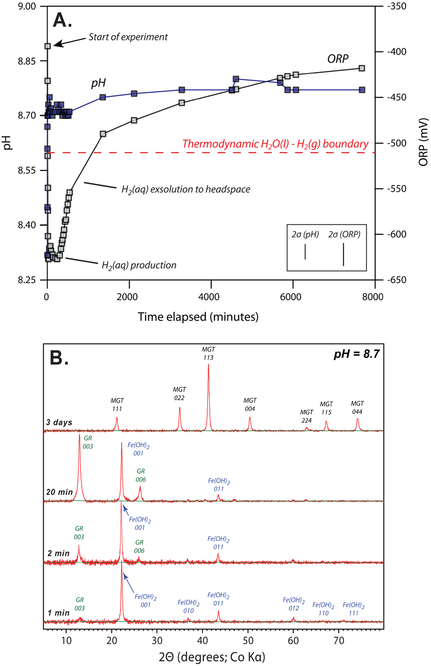
Fig. 1.
Anoxic precipitation experiment with Mg2+, Fe2+, and SiO2(aq) bearing water. (A.) As pH is increased oxidation-reduction potential (ORP) falls below the thermodynamic stability of H2O(l), as H2(aq) is generated, which then slowly degasses to the reactor headspace. (B.) Under strictly anoxic conditions, pH increases precipitate Fe(OH)2 which rapidly converts to green rust, in turn reducing H2O(l) and forming H2(aq). Metastable green rust transforms to magnetite in days. Error bars denote 2σ derived from triplicate experiments.