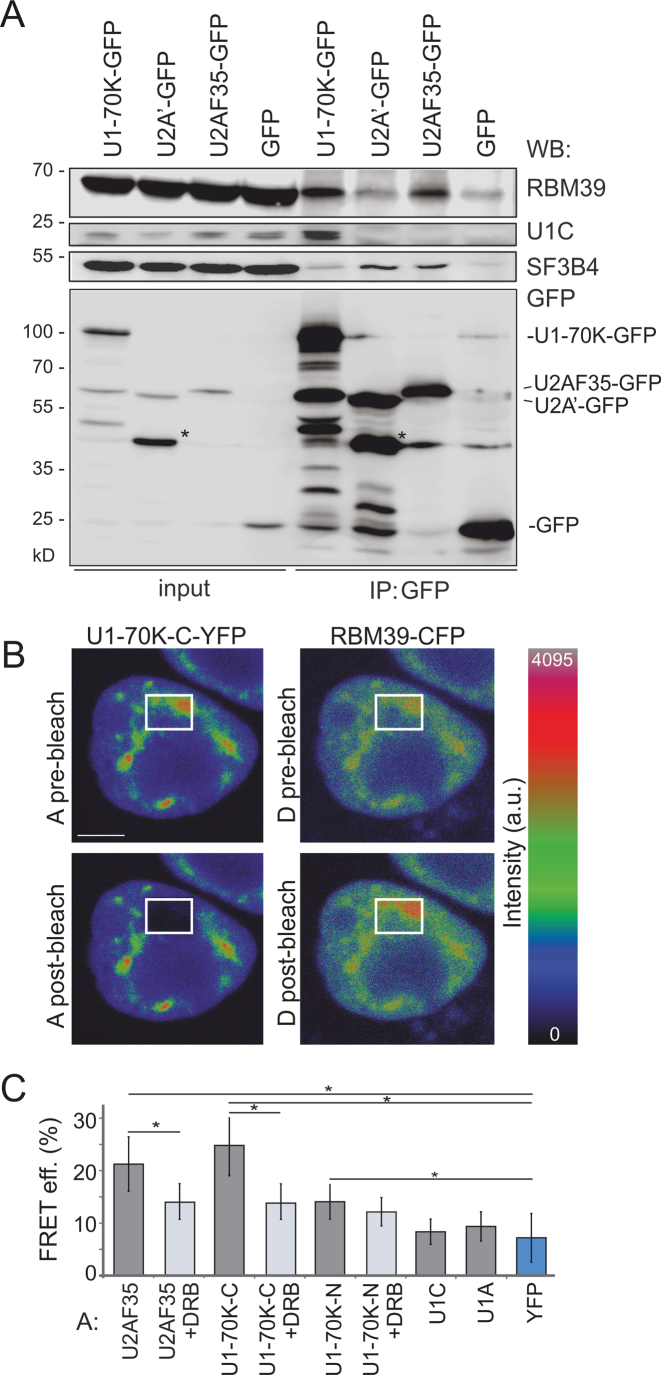
Figure 5.
RBM39 interactions with spliceosome components. (A) RBM39 interacts with U1 snRNP and U2AF. Interaction of RBM39 with the U1-specific protein U1-70K, the U2-specific protein U2A’ and the small subunit of U2AF was assayed by immunoprecipitations. HeLa cells were transiently transfected with U1-70K-GFP, U2A′-GFP or U2AF35-GFP, immunoprecipitated with anti-GFP antibodies and probed with antibodies shown to the right. U1C and SF3B4 served as positive controls for immunoprecipitations for U1-70K-GFP and U2A’-GFP, respectively. Asterisks denote a partially degraded U2A’-GFP. (B, C) RBM39 interactions monitored by FRET. Cells were transiently co-transfected with RBM39-CFP and C-terminally YFP-tagged U1-70K. (B) YFP was bleached in a small region comprising the nucleoplasm and nuclear speckles; CFP fluorescence was measured before and after bleaching. Fluorescence of RBM39 increased after bleaching of U1-70K-YFP [cf. CFP fluorescence in the bleached region (rectangles) before (top panel) and after (bottom panel) bleaching]. A, acceptor; D, donor; scale bar, 5 μm. (C) Quantification of individual donor-acceptor FRET efficiencies upon the inhibition of RNA polymerase II by DRB. Columns indicate means; errors bars SEMs. Interaction between RBM39-CFP and U2AF35-YFP (22) served as a positive control and interaction between RBM39-CFP and YFP as a negative control. Significantly different means are denoted by an asterisk (P< 0.01; t-test).