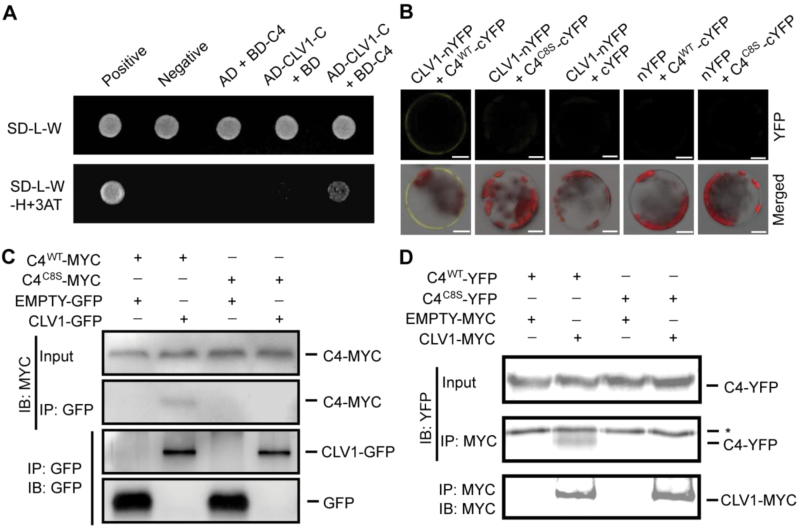
Fig. 5.
S-acylation of C4 is important for its interaction with CLV1. (A) Detection of the interaction between C4 (fused to the binding domain, BD) and the intracellular domain (704AA-967AA) of CLV1 (CLV1C; fused to the activation domain, AD) in a yeast two-hybrid assay. Protein interactions were tested using a stringent (SD/–Leu/–Trp/–His) selection medium containing 3-amino-1,2,4-triazole (3AT). (B) The in vivo interaction between C4 and CLV1 was measured using bimolecular fluorescence complementation assays. The combinations with empty vectors were used as negative controls. The YFP signals and merged signals (yellow for YFP, red for chloroplast autofluorescence, and gray for bright field) are shown. Scale bars are 10 µm. (C) Detection of the interaction between C4-MYC and CLV1-GFP using co-immunoprecipitation (Co-IP). The wild-type (WT) or mutant form of pCanG-C4-MYC (C8 mutated from a cysteine to a serine) was co-transformed with 35S::CLV1-GFP or 35S::GFP (control). After 48 h, the transformed protoplasts were collected for Co-IP using GFP-Trap resin. (D) Measurement of the interaction between C4-YFP-FLAG3His6 and CLV1-MYC using Co-IP. The wild-type or mutant form of C4-YFP-FLAG3His6 was co-expressed with pCanG-CLV1-MYC or pCanG-MYC (control). Co-IP was performed using an anti-MYC antibody with Protein A resins. * indicates the unspecific signal from the antibody. The results in this figure are representative of independent experiments.