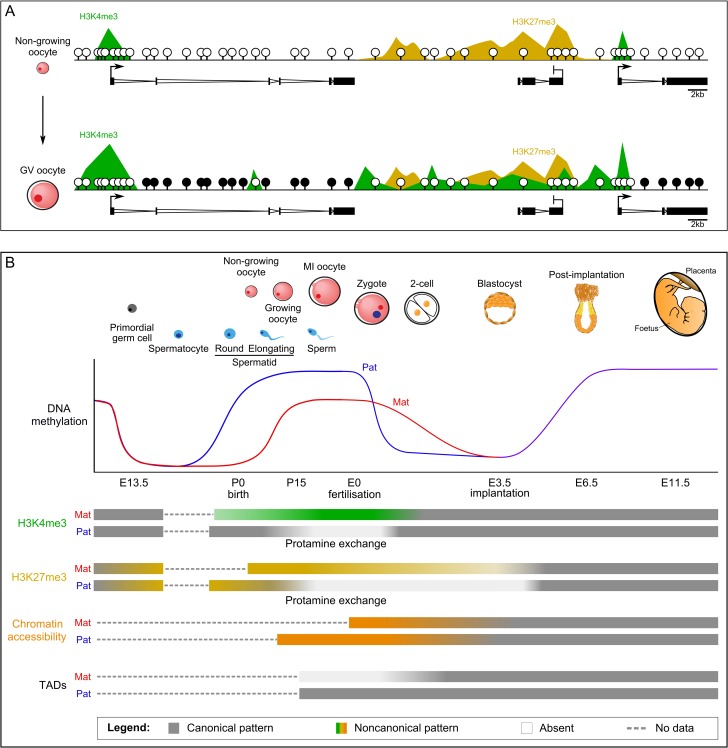
Figure 3.
Epigenetic reprogramming in mouse development. (A) Epigenetic patterns are shown for non-growing oocytes and fully grown germinal vesicle (GV) oocytes. In non-growing oocytes, DNA is almost completely unmethylated, H3K4me3 is exclusively enriched at active promoters and H3K27me3 is spanning broad non-canonical domains. By the fully grown GV stage, DNA across transcribed gene bodies is fully methylated and H3K4me3 has accumulated in broad domains throughout untranscribed regions. (B) Schematic of epigenetic reprogramming events during gametogenesis and embryogenesis. DNA methylation is erased in primordial germ cells and re-established earlier in the sperm of males and after birth in oocytes in females. Oocytes acquire lower overall methylation than sperm, with non-canonical genome-wide distribution. After fertilisation, the paternal DNA is rapidly demethylated, while maternal DNA methylation is passively lost over several cell divisions. DNA methylation is re-acquired in canonical patterns in the post-implantation embryo, concomitant with lineage specification. H3K4me3 is non-canonically distributed in the oocyte, is rapidly erased after fertilisation, and becomes canonically enriched at CpG islands and active promoters. Very few domains retain H3K4me3-marked histones in the protamine exchange in sperm and subsequently through the re-acquisition of histones in the zygote. H3K27me3 acquires a non-canonically broad distribution in PGCs in the absence of other repressive epigenetic marks. This pattern is relatively maintained throughout oogenesis, while very few H3K27me3-marked histones are retained in the sperm protamine exchange. In the pre-implantation embryo, H3K27me3-transmitted from the gametes is progressively lost, with pronounced loss at CpG-rich regions. H3K27me3 is then re-established in a canonical pattern in the post-implantation embryo. Chromatin accessibility is contrastingly and exceptionally open in the oocyte and compact in the sperm. The open chromatin state of maternal DNA is gradually resolved in the pre-implantation embryo, while the compact packaging of paternal DNA is rapidly resolved with incorporation of histones in the zygote. Topological associated domains (TADs) are nearly absent in the mature oocyte and become gradually re-instated in the pre-implantation embryo.