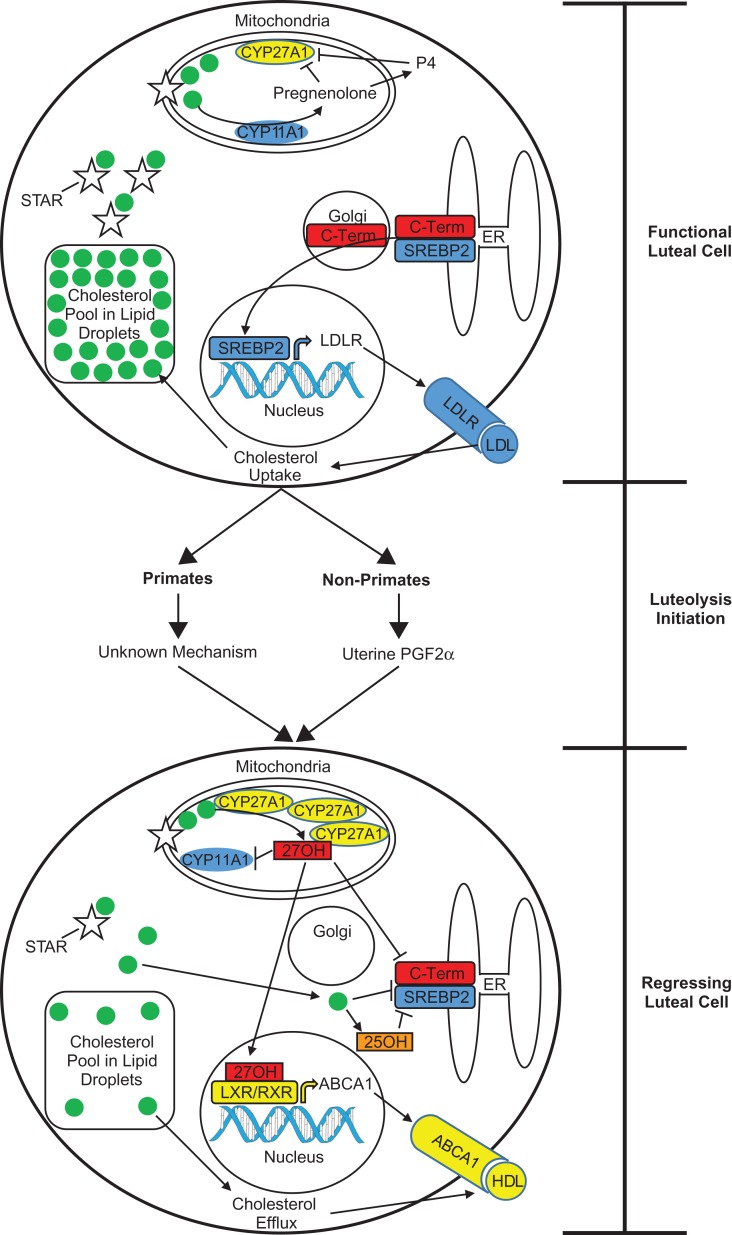
Figure 6.
Proposed model of luteolysis in primate and non-primate species. During the luteal phase, functional luteal cells have a large reserve of cholesterol stored in lipid droplets. Free cholesterol is transported into the mitochondria via STAR, and converted into pregnenolone by CYP11A1 (aka, p450 side-chain-cleavage). Pregnenolone leaves the mitochondria and is converted to P4, and both steroids inhibit the enzyme activity of CYP27A1. Because cholesterol is effectively sequestered by lipid droplets and STAR, SREBP2 is transported from the endoplasmic reticulum (ER) to the Golgi where the c-terminal regulatory region is cleaved. The active transcription factor travels to the nucleus and stimulates LDLR transcription, ultimately resulting in increased LDL uptake and maintenance of cholesterol reserves. Luteolysis is initiated by uterine-derived PGF2α in non-primate species, whereas the mechanism initiating luteolyis in primates is not known. Regardless of the differences in the initiation of luteolysis, the mechanisms mediating the regression of luteal cells may be similar. Elevated CYP27A1 protein concentrations in mitochondria cause increased conversion of cholesterol into 27OH, which subsequently inhibits SREBP2 proteolytic processing and is an agonist for LXR/RXR (retinoid x receptor) heterodimers. This results in increased transcription of LXR target genes including ABCA1. There may also be direct inhibition of CYP11A1 enzymatic activity and steroidogenesis by mitochondrial 27OH. Additionally, decreased STAR expression contributes to reduced cholesterol sequestration and increased association of free cholesterol with the ER. This prevents SREBP2 proteolytic processing, possibly due to cholesterol binding to SCAP and/or increased 25OH synthesis from the ER-localized cholesterol 25-hydroxylase enzyme. The net result of impaired LDL uptake and enhanced cholesterol efflux is a depletion of cholesterol reserves and a gradual loss in steroidogenic capacity.