ABSTRACT
We previously investigated MET and its oncogenic mutants relevant to lung cancer in C. elegans. The inactive orthlogues of the receptor tyrosine kinase Eph and MET, namely vab-1 and RB2088 respectively, the temperature sensitive constitutively active form of KRAS, SD551 (let-60; GA89) and the inactive c-CBL equivalent mutants in sli-1 (PS2728, PS1258, and MT13032) when subjected to chronic exposure of nicotine resulted in a significant loss in egg-laying capacity and fertility. While the vab-1 mutant revealed increased circular motion in response to nicotine, the other mutant strains failed to show any effect. Overall locomotion speed increased with increasing nicotine concentration in all tested mutant strains except in the vab-1 mutants. Moreover, chronic nicotine exposure, in general, upregulated kinases and phosphatases. Taken together, these studies provide evidence in support of C. elegans as initial in vivo model to study nicotine and its effects on oncogenic mutations identified in humans.
KEYWORDS: C. elegans, MET, MT13032, PS2728, PS1258, RB2088 and nicotine, SD551, vab-1
Introduction
Lung cancer is a devastating disease that afflicts more than 200,000 people per year in United States alone and the associated number of deaths is more than any other type of cancer. Cigarette smoking is the major underlying etiological factor. Although cigarette smoke contains more than 60 carcinogens, nicotine, a major component of cigarette smoke, is a cocarcinogen, and the principal addictive agent.1,2 The nicotine-derived nitrosamines are nevertheless carcinogenic. Moreover, nicotine is known to promote cancer cell proliferation, angiogenesis and epithelial-mesenchymal transition thereby aiding tumor growth and metastasis.3-5 The conduits for nicotine mediated signaling are the ubiquitously expressed acetylcholine receptors (nACHR) whose downstream signaling targets are also common to pathways mediated by receptor tyrosine kinases (RTK).6-8
The simple soil nematode, C. elegans, is gaining acceptance as an in vivo model to investigate the toxic effects of metals and pesticides.9 In addition, highly addictive substances such as nicotine, alcohol, cocaine and opioids have also been investigated in this animal model.10-24 The end points in the investigations are usually changes in the worm behavior (locomotion, chemotaxis, feeding), shape (morphological), development (fork head, multiple vulva) and genome (DNA damage). Its fully sequenced genome combined with short life cycle, small size and a highly invariant cell lineage make it an excellent model to investigate the role of a variety of key signaling pathways. It also has the added advantage of having minimal molecular redundancy in a variety of signaling pathways.
RTKs contribute significantly to the oncogene addiction seen in cancers.25-27 In this regard, we and others have shown that the RTKs such as EGFR, MET, RON and Eph are not only overexpressed but frequently undergo gain-of-function mutations in a variety of cancers.26,28-31 Our continued effort to identify potential lung cancer therapeutic targets led us to the discovery of increased occurrence of gain-of-function mutations in RTKs such as MET, EphB4, key intracellular signaling molecules such as RAS GTPase, the adapter focal adhesion molecule Paxillin and the PAX transcription factors 5 and 8.32-34 Their downstream target such as RAS is also known to undergo oncogenic mutation. On the other hand, mutations in c-CBL, the E3 ubiquitin ligase that negatively regulates RTKs, characterized in lung cancer, turned out to promote cell growth and proliferation and enhance cell motility. The c-CBL wild type (wt), however, suppressed the above effects.35 Moreover, we have previously demonstrated that C. elegans can be used as an in vivo model for the rapid screening MET gain-of-function mutants discovered in human lung cancer. Transgenic worms that expressed either MET R988C or MET T1010I suffered from significantly low fecundity and abnormal vulval development characterized by hyperplasia compared to those worms that expressed wt MET. Interestingly, the above effects were exacerbated by nicotine treatment.36
Our search of the C. elegans data base revealed the existence of strains that harbored non-functional mutants of MET (RB2088), Eph (vab-1(e2)II), RAS (SD551 derived from let-60) and c-CBL (PS2728, PS1258, MT13032 derived from sli-1). Since the RTKs MET and Eph, and the RAS GTPase are known to be over expressed in several cancers and support cell proliferation and growth, we hypothesized that non-functional mutants of these molecules will adversely affect the survival and related functions in the worms. In comparison, the wt c-CBL negatively regulates RTKs, and worms harboring nonfunctional sli-1 are therefore expected to have abnormal growth and motility. Based on our previous work, we also postulated that chronic exposure to nicotine would further aggravate the various functional behaviors in the above mutant strains.36
The current studies are aimed at understanding the effect of chronic exposure to nicotine on the phenotype, survival, fertility, egg-laying capacity, locomotion and gene expression profiling on select C. elegans mutants relevant to cancer. The mutant worms used were vab-1 (inactive, Eph), RB2088 (inactive, MET), SD551 (temperature sensitive strain expressing constitutively active form of KRAS), and 3 sli-1 mutants PS2728, PS1258, and MT13032 (inactive, c-CBL). Here we report the changes in the intrinsic behavioral and functional aspects of the above mutants compared to wt N2 control animals. Also, we carried out gene expression profiling studies. Finally, we also present here the effects of chronic nicotine treatment on the above mutants that support our contention that C. elegans is a suitable in vivo model to screen functionality of cancer mutations.
Methods
Nematode strains and culturing
The Bristol N2 strain was used as a wt standard in all the experiments. Non-functional mutant strains used in this study were vab-1(eII)II (G912E; Eph ortholog), RB2088 (MET equivalent), and sli-1 (c-CBL ortholog) mutants: PS2728, PS1258 and MT13032. In contrast, SD551 harbors a temperature-sensitive mutant of a KRAS ortholog. It is inactive at 15°C but active at 20°C, the temperature at which the worms were cultured.Table 1 is a summary of the various mutants used in this study. The worms were cultured on standard nematode growth medium (NGM) agar plates carrying a lawn of E. coli OP50 as a food source at 20°C as previously described.37 All the strains were obtained from the Caenorhabditis Genetic Center at the University of Minnesota.
Table 1.
Various strains of C. elegans used in the study explaining their genotypes and phenotypes.
| Strain ID | C.elegans Ortholog | H.Sapiens Ortholog | Genotype | Variation | Source | Phenotype | Notes |
|---|---|---|---|---|---|---|---|
| N2 | — | — | — | — | CGC | — | WT Bristol Variant |
| SD551 | let-60 | Ras family | let-60(ga89) IV | unknown | CGC | varied,primarily Muv | Exons 6–11 missing (most of kinase domain) |
| CB2 | vab-1 | Eph family | vab-1(e2) II | 2735G>A | CGC | notched head | <70% penetrance |
| RB2088 | F11E6.8 | Met family | F11E6.8(ok2754) IV | 900-bp deletion | CGC | unknown | |
| MT13032 | sli-1 | Cbl family | sli-1(n3538) X | 914C>T (S305L) | CGC | SynMuv | — |
| PS2728 | sli-1 | Cbl family | sli-1(sy143) X | 454C>T (Q152*) | CGC | SynMuv |
Reagents
Nicotine (cat. #N0267), 5-Fluoro-2′-deoxyuridine, (FUDR) (cat. # F0503), Ampicillin (cat.# A1593) were obtained from Sigma. Trizol was obtained from Life Technologies (cat. #13596–06).
Kaplan-meyer survival assays on NGM agar
NGM agar plates were prepared with Amp/FUDR to which nicotine was added at various concentrations (control, 50 μM and 500 μM) according to the protocol (http://www.jove.com/index/details.stp?ID=1152). For each group, 2 plates were used and on each plate 20 – 25 age synchronized L4 worms were transferred and the worms were grown at 20°C. Every two days the worms were transferred to a new NGM agar plate. The worms were counted every 12 h for 2 weeks. Survival curves were plotted using the survival package for the R Statistical Software.38-40
Egg-laying assay
Worms were grown from synchronized egg populations on nicotine and control plates to early L4 stage and 4 worms were then transferred to a single well in a 24-well tissue culture plate with identical NGM agar composition.41,42 Newly laid eggs were counted after 6.0 h from all the wells. The rate of egg-laying was calculated as eggs/worm. For each strain the assay was repeated 3 to 4 times.
Fertility assay
Eggs were isolated from gravid adult hermaphrodite worms by alkaline hypochlorite treatment and then grown on the NGM agar plates with and without nicotine till early L4 stage. Single L4-stage worms grown at 20°C were transferred to fresh plates every day until they stopped laying eggs. All progeny plates were incubated at 20°C for 2 days, and the number of progeny developed was counted for every plate.42 The assay was repeated for each strain at least 3 to 4 times.
Liquid culturing of C. elegans for phenotypic study
The worms were grown in 24-well tissue culture plate according to the method of Fitzgerald.43 In brief the liquid culture was prepared with sterilized S basal buffer (5.85g NaCl, 1g K2HPO4, 6 g KH2PO4 and water to 1 L) supplemented with OP50 E. Coli bacteria, cholesterol, FUDR (25μM) to suppress reproduction along with nicotine. Synchronized L4 worms were added at a density of 12 ± 6 worms per well. Liquid culture was changed daily by carefully aspirating the liquid. The plates containing worms were kept on orbital shaker at room temperature. The experiment was carried out for 10 d. The worms were monitored daily and photographed for any phenotypic changes using inverted microscope (Olympus IX71, Center valley, PA, USA).
Locomotion analysis
Locomotion behavior was analyzed using an automated worm tracking system as previously described.24,44 In brief, eggs were isolated from adult worms by alkaline hypochlorite treatment and grown on NGM agar plates with and without nicotine to obtain synchronized L4 population. For analysis, 20 L4 worms were transferred to 35 mm NGM agar bacteria free plate with an identical agar composition to the original culture plate. The experimental worms were then transferred to fresh plates one hour before the analysis so as to get them acclimatized. For each condition, we used 5 such plates. The temperature was maintained at 20–21°C with relative humidity of 30–40%. Behavior was recorded for 10 minutes at 2 frames per second using a CCD camera (Prosilica GC2450, Allied Vision Technologies, Stadtroda, Germany). Movies were analyzed using a modified version of existing MATLAB (MathWorks, Natick, MA) scripts.45 After background subtraction, individual animals were detected based on a pixel intensity threshold and particle size and their centroid coordinates determined for each frame. Speed for each worm was computed as mean centroid displacement (mm) per second excluding regions where the animal made sharp-angle turns.
Gene expression analysis
Eggs were isolated using hypochlorite treatment and grown on control and nicotine containing plates till early L4 stage. A total 5, 10 cm NGM agar plates, with and without nicotine, were used for each group. Once the worms became adults, total RNA was extracted from all the strains using Trizol. RNA extraction was done from 3 independent experiments Global gene expression analysis was determined by microarray analysis of the extracted RNA as described below.
Gene expression microarray experiments were conducted with biological replication in all samples. Sample processing order was randomized. RNA quality was assessed by Bioanalyzer (minimum RIN = 7.5). cRNA was produced using the Agilent Low-Input Linear amplification and labeling kit. Array hybridizations (Agilent C. elegans (V2) Gene Expression Microarray, 4x44K) were performed at the University of Chicago and Argonne National Labs high throughput genome analysis core facility, according the manufacturer’s instructions. The Agilent FE software was used to extract feature intensities and to flag saturated, non-uniform and outlier features. Probe intensity was adjusted by subtracting background intensity using the minimum method and quantile normalized between arrays.46 Outlier arrays were eliminated based on total number of flagged probes, intra-array variance, inter-array variance, biological replicate variance and spike-in linearity. Multiprobe probe sets were hierarchically clustered. Using one minus the Pearson correlation coefficients as a distance matrix, clusters were divided into groups by cutting clusters at a dendrogram height of 0.5 (roughly producing clusters with internal correlation coefficients 0.5). All downstream analyses were performed independently on each resulting cluster and all single probe probesets. For each probe set, surrogate variable analysis (SVA) was performed on the matrix of expression measurements, after controlling for the effects of genotype and nicotine exposure.47 For each probe set, we then constructed a linear fixed effects model y ˜ m + (genotype * nicotine) + probe + e, where y is the log2 transformed probe intensity, m is the expected probe intensity, genotype is a factor representing the effect of genotype, nicotine is a factor representing the effect of nicotine and probe the effect of the oligonucleotide probe and e is the residual error. The * represents the genotype and nicotine effects are fully crossed, to identify interactions between genotype and nicotine. The significance of covariate effects was assessed by estimating false discovery rates, using Storey’s q-value method.48 Differentially expressed genes, defined by FDR cutoff (see above) were entered into DAVID (the database for annotation, visualization and integrated discovery) bioinformatics resources.49,50 All genes on the array were used as a background.
Statistical analysis
Cox proportional hazards model was used to assess the effect of nicotine and strains on survival. For egg laying and fertility assays, we estimated the effect of nicotine and variant using mixed effects linear model with treatment and variant type and their interaction terms as covariates. A random effect for batch was used to account for the fact that some batches tend to be more productive than others.
Locomotion data was analyzed using speed as outcome and treatment as a categorical covariate. Regular, log-transformed and robust regression approaches were used to assess sensitivity of results to outliers and distributional assumptions. Analysis of locomotion was done according to a 2 sample z test which is a standard hypothesis test comparing 2 means. The absolute value of the z score had to be greater than 1.96 to corresponds to the p = 0.05 significance level that was used. Otherwise, both means were considered to be the same.
Results
Chronic nicotine treatment decreases C. elegans survival
Survival assays were carried out over a period of 2 weeks on worms grown in NGM agar plates that were exposed to nicotine (50 μM and 500 μM). As shown in Fig. 1, chronic exposure to nicotine, especially at higher concentration, had significant impact on survival of all strains of worms except for the 2 sli-1 mutants (MT13032 and PS1258). The vab-1 (e2)II and SD551 strains suffered the maximum loss in survival compared to the wt worms and other mutant strains. Treatment with nicotine reduced the lifespan of worms for all strains (p = .0034). Survival also depends on strain, with N2 being the longest living group (p = .0004). We did not find significant difference in nicotine treatment effect on survival across different strains. Since nicotine is known to induce behavioral changes in worms,23 we next determined the effects of nicotine treatment on egg-laying capacity, fertility and locomotion.
Figure 1.
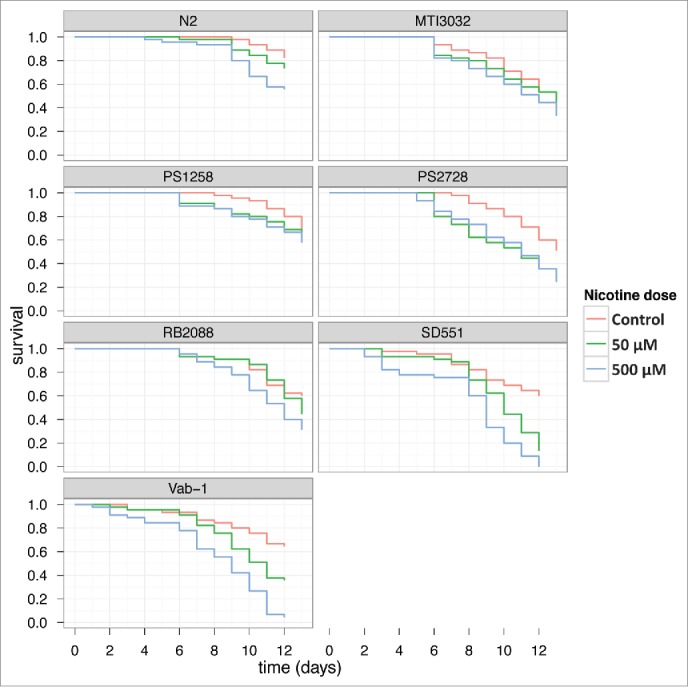
Effect of Nicotine on survival ofC. elegansSurvival plots of C. elegans when exposed to different concentrations of nicotine are shown. The experiment was repeated twice and the results were comparable. The data was subjected to Kaplan Meier survival analysis using Graphpad Prism Software 4.02V.
Nicotine suppresses egg-laying capacity and fertility of C. elegans
The average number of eggs laid by L4 worms during a 6 hour period after 10 d of nicotine treatment is presented in Fig. 2A. Except for RB2088 and SD551, the egg-laying capacity of all other strains was somewhat lower as compared to N2 worms. Nicotine treatment had a suppressive effect on the egg-laying capacity of all strains, including that of control worms, reducing egg production by 9% at 50 μM nicotine and 27% at 500 μM nicotine. As shown in Fig. 2A, the rate of nicotine-induced decrease in egg-laying capacity was affected significantly more in the sli-1 mutant strain PS2728 (p = .0091) and suggestively more affected in the MET mutant equivalent strain RB2088 (p = .0643).
Figure 2.
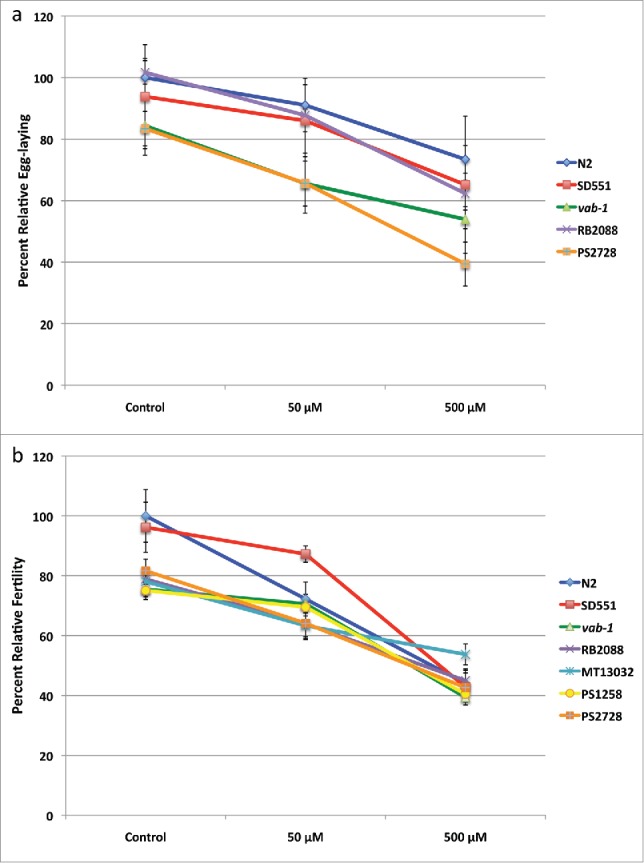
Effect of Nicotine on egg-laying capacity and fertility ofC. elegansa. Egg-laying responses to chronic exposure of nicotine were assayed on NGM agar. Each condition had 24 worms and the assay was repeated 4 times. Rate at which eggs were laid was calculated as eggs/worm and percent relative egg-laying compared to untreated N2 worms is shown. b. To quantify the number of progeny produced by hermaphrodites, synchronized L4 worms were collected and at least 4 worms were allowed to lay eggs on individual plates. Animals were examined until no progeny were produced within a 24-h period and percent relative fertility compared to untreated N2 worms is shown.
We next determined the total number of progeny generated in a worm’s life span for each of the strains. Fig. 2B shows percent relative fertility compared to the N2 control. Overall, the fertility of most of the untreated mutant strains was lower compared to N2 worms by about 20% except for SD551. With increasing concentrations of nicotine, the percentage fertility of each strain decreased. At the highest concentration of nicotine, differences between the untreated strains was completely abrogated. N2 and SD551 were the most affected strains, showing maximum reduction in fertility upon nicotine exposure.
Nicotine induces morphological changes in C. elegans
We next wanted to know whether the chronic exposure of nicotine has any effect on the morphology of these mutants. The RAS mutant SD551 revealed multivulva phenotype that was exacerbated upon chronic exposure to nicotine (Fig. 3a and b). Meanwhile, the sli-1 mutant strains, PS2728 and MT13032, had a dramatic decrease in body size as compared to untreated control worms (Fig. 3c and d). Percentage of SD551, PS2728 and MT13032 worms with phenotypic changes in the presence and absence of nicotine are summarized in Table 2. Treatment with nicotine significantly increased morphological changes in each of the mutant strains compared to control (p<.05). However, exposure to nicotine did not induce any morphological changes in N2 (data not shown).
Figure 3.
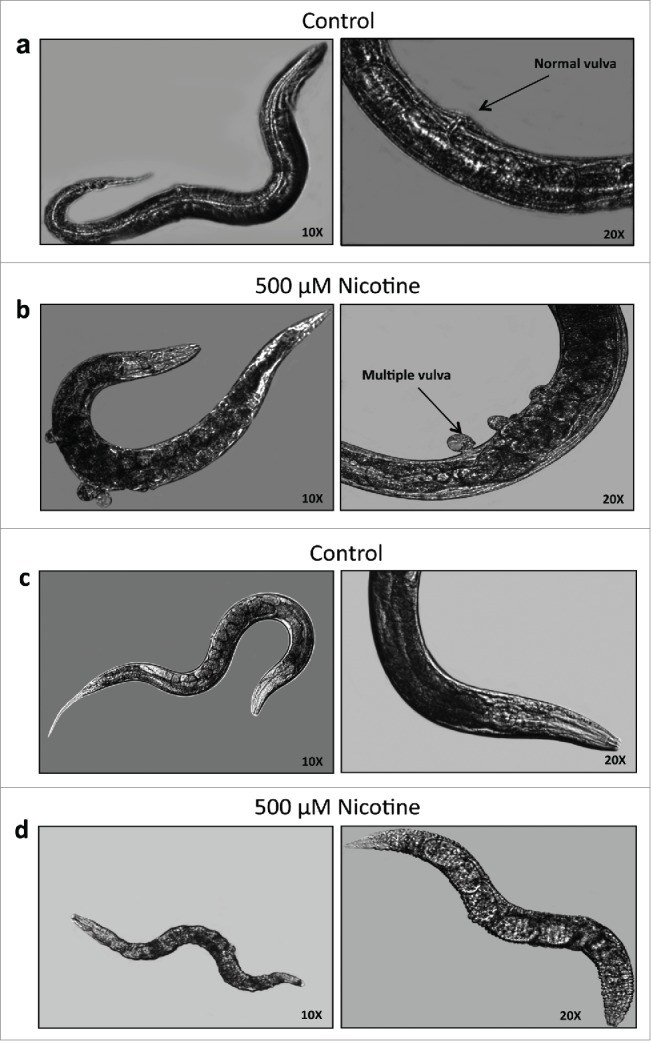
Phenotypic changes inC. elegansafter chronic treatment with nicotine. Phenotypes of SD551 mutant. 10X and 20X magnification images of SD551 a. control showing normal vulva phenotype and b. with nicotine showing multiple vulva phenotype. Phenotypes of PS2728 and MT13032 (Sli-1) mutants. 10X and 20X magnification images of PS2728 c. control showing normal body size and d. with nicotine showing shrinkage in the body size (MT13032 not shown).
Table 2.
% of C. elegans phenotypes observed with chronic nicotine treatment.
| Strain | Control | 500 μM Nicotine | 1000 μM Nicotine |
|---|---|---|---|
| PS2728 | 2.22 ± 2.22 | 10.74 ± 3.53 | 11.85 ± 2.67 |
| MT13032 | 0.00 ± 0.00 | 7.04 ± 1.61 | 9.26 ± 3.76 |
| SD551 | 1.11 ± 1.11 | 11.11 ± 5.13 | 12.59 ± 4.51 |
Synchronized L4 worms of all the strains were exposed to nicotine in liquid culture for 10 d and their phenotypes were observed. Values are given as Average ± SEM. There is a significant difference between the % phenotypes of control, 500 μM and 1000 μM nicotine within each strain (p < 0 .05)
Chronic exposure to nicotine enhanced locomotion in C. elegans
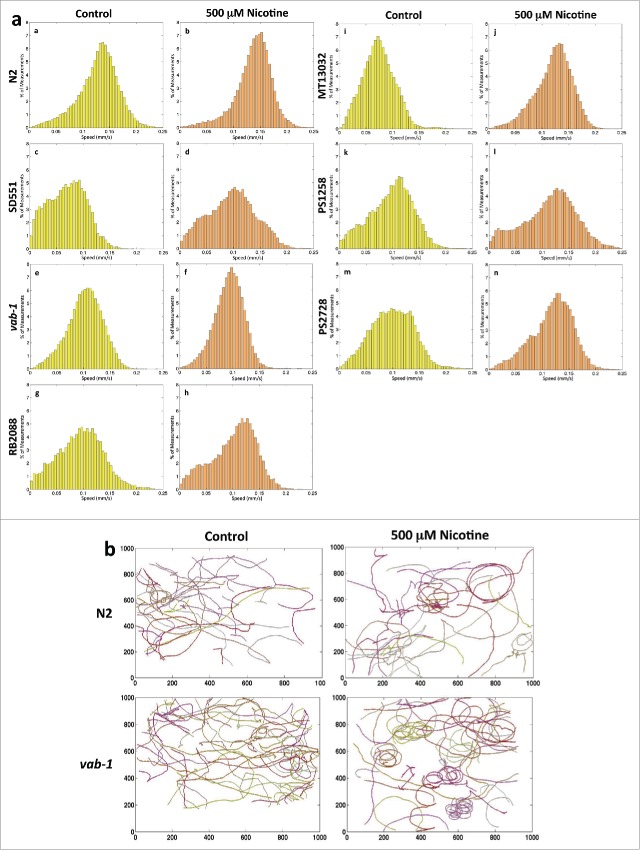
In the absence of nicotine, there is a significant decrease in speed of all mutant worms compared to the wt N2 worms (Fig. 4a). Our data shows that N2 worms had maximum average speed while SD551 had minimum average speed in comparison to other mutants. Notably, chronic exposure to nicotine significantly enhanced the locomotion speeds in wt and all mutants except vab-1 (Fig. 4a). The relative distribution of speeds is shown for all the worms and the average speeds before and after nicotine treatment in the various groups of worms tested are summarized in Table 3. We also found that vab-1 mutant showed increased circular motion compared to the wt N2 (Fig. 4b, supplementary videos 1, 2, 3, 4).
Figure 4.
(A). Effect of chronic exposure of nicotine on the locomotion velocity of differentC. elegansstrains. Synchronized worms were grown on plates with and without nicotine. 20-30 L4 worms were transferred to bacteria free plates with nicotine and incubated for 20 min before recording tracks. Worms were tracked for 600 seconds. The corresponding normalized histograms of N2, SD551, vab-1, RB2088, MT13032, PS1258 and PS2728 speeds with (a, c, e, g, i, k, m) and without nicotine (b, d, f, h, j, l, n) are respectively shown. Average velocity (mm/s) of each mutant is shown in Table 3. (B). Effect of chronic exposure of nicotine on worm path. Worms (N2 wt, and vab-1) were tracked for 10 minutes. The tracks of 20 worms with and without nicotine are shown.
Table 3.
Effect of chronic exposure of nicotine on locomotion speed (mm/s) of different mutants.
| Strain | Control | Nicotine 500 μM |
|---|---|---|
| N2 | 0.13134 + 0.00018 | 0.13955 + 0.00016 |
| SD551 | 0.07222 ± 0.00028 | 0.09903 ± 0.00035 |
| vab-1 | 0.10525 + 0.00016 | 0.09523 + 0.00012 |
| Rb2088 | 0.09740 ± 0.00036 | 0.10451 ± 0.00036 |
| MT13032 | 0.07693 ± 0.00015 | 0.12419 ± 0.00014 |
| PS1258 | 0.10011 ± 0.00029 | 0.11642 ± 0.00029 |
| PS2728 | 0.10188 ± 0.00028 | 0.12069 ± 0.00023 |
With exposure to nicotine, locomotion speed of all the strains has increased except for vab-1. N2 has maximum speed while SD551 has the least. Values are given as Average ± SEM.
Chronic exposure to nicotine and gene expression analysis in C. elegans
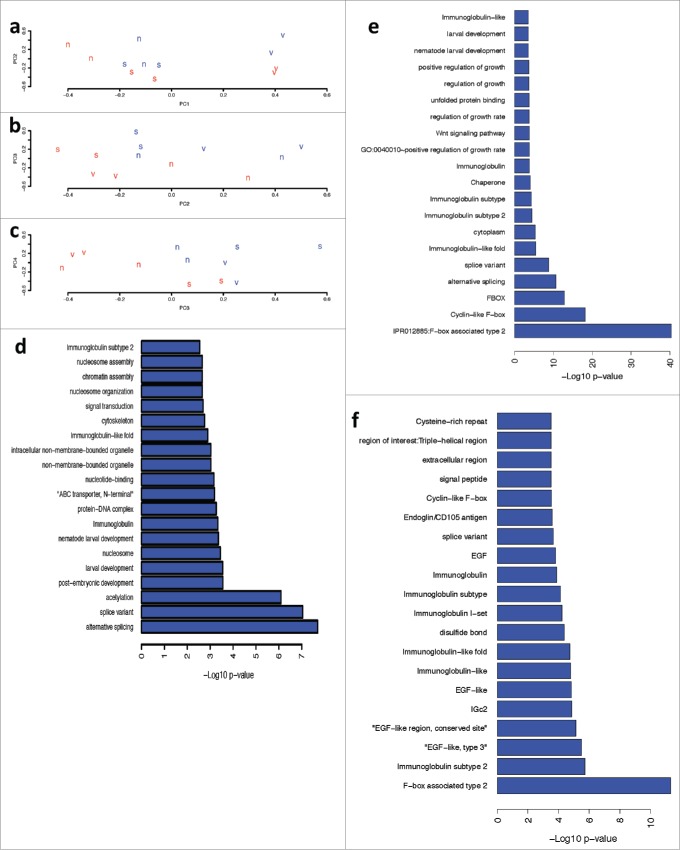
The gene expression experiments focused on the Eph receptor mutation (vab-1) and the RAS mutation (SD551) with nicotine concentrations of 0 and 500 μM. The linear models used in these experiments provide differentially expressed genes for each genotype, for nicotine alone, and for the interaction between nicotine and each genotype. Mutation of vab-1 dramatically altered gene expression patterns, with 4496 genes differentially expressed (FDR < 0.01). SD551 mutations also significantly altered gene expression, with 1276 genes significantly differentially expressed (FDR < 0.01). Nicotine had a smaller effect, affecting the expression of 986 genes (FDR < 0.05). This can also be seen in the principal components analysis; the first 2 principal components (Fig. 5a) largely separate the samples based on genotype (although the second PCA does also show separation based on nicotine exposure). Interestingly, however, the normal genotype exposed to 500 μM nicotine clusters with the RAS mutant worms (Fig. 5, 5b), suggesting that, at least with regards to global gene expression, exposure of normal worms to nicotine phenocopies mutation of RAS (Fig. 5c). PCAs 2–4 (Fig. 5b, c) separate samples based on nicotine exposure.
Figure 5.
Effect of Nicotine on Gene Expression analysis of N2,vab-1and SD551 mutants ofC. elegans. Panels a-c: Principle Components Analysis plots of the first 3 PCAs. The letter designates the genotype of the strain (v = vab-1, s = SD551, n = N2) and the color designates nicotine exposure (blue = 500 x nicotine, red – 0 x nicotine). Panels d-f: -log10 p-value (fisher’s exact test) for enrichment of pathways and process in differentially expressed genes. Panel d shows nicotine alone, Panel e show the interaction between nicotine and vab-1, and Panel f shows the interaction between nicotine and SD551.
To understand the biological process affected by mutation of Eph, RAS and exposure to nicotine, we performed pathway analysis on differentially expressed genes. Mutation of Eph significantly altered several important processes, including cell cycle, embryonic development and larval development (Supplementary Fig. 1). Mutation of RAS altered a huge number of F-box containing proteins (Supplementary Fig. 2). C. elegans had an expansion of F-box proteins during evolution and the function of many of these proteins is poorly understood. RAS also significantly affected the expression of several serine-theronine kinases, as well as structural genes and genes involved in locomotion and response to stimulus. Nicotine exposure by itself significantly affected important pathways, such as post-embryonic and larval development and signal transduction (Fig. 5d). The interaction of Eph and nicotine led to altered expression of numerous F-box proteins as well. However, it also led to differential expression of several interesting classes of genes, including positive regulation of growth rate (Fig. 5e). A heat map of the linear model effect size of the 280 genes (Supplementary Table 1) in positive regulation of growth rate shows that mutation of Eph and, to a lesser extent, exposure to nicotine, drives gene expression in the same direction. Interestingly, however, the interaction between Eph and nicotine strongly drives gene expression in the opposite direction. As specific examples, expression of Paxillin and EGL-15 (i.e. FGFR homolog) are strongly increased by mutation of Eph, and their expression is downregulated in EphA mutants exposed to nicotine. Alternatively, PAR-6, a gene involved in epithelial cell polarity, is downregulated by Eph and upregulated by the combination of Eph and nicotine. This is in contrast to several serine-threonine kinases that are significantly differentially expressed in both Eph and RAS mutants (Fig. 6a–f). Most kinases are strongly upregulated by mutations and by nicotine, and even more strongly upregulated by the interaction between genotype and nicotine.
Figure 6.
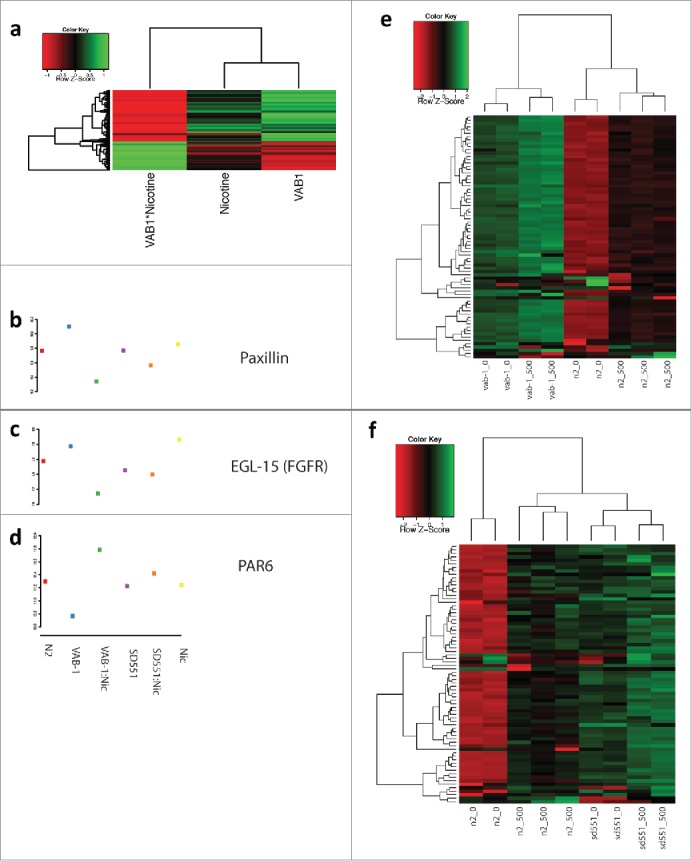
Effect of Nicotine on heatmap analysis of N2,vab-1and SD551 mutants ofC. elegans.Panel a: Heatmap of β effect sizes from linear models for differentially expressed genes in the “positive regulation of growth rate." Panels b-d: Beta effect sizes for 3 example genes in “positive regulation of growth rate." (Red = N2, Blue = vab-1, Green = vab-1+500 Nicotine, Purple = SD551, Orange = SD551+Nicotine, Yellow = 500 Nicotine). Panels e-f: Heatmap of gene expression levels for differentially expressed serine-threonine kinases.
Given the phenotypes above, we next asked whether expression of genes known to have similar phenotypes when mutated was altered by mutation of RAS or Eph, or addition of nicotine. For example mutation of Eph altered expressed of 54 UNCoordinated genes, and the interaction between Eph and nicotine altered expression of 18 UNCoordinated genes. Expression of Egg-Laying (egl) defective genes were also altered by mutation of Eph (18 genes) and RAS (11 genes). Interestingly, although mutations of RAS generated a multi-vulva phenotype that was exacerbated by exposure to nicotine, only 1 of the 38 genes known to have a SynMuv phenotype demonstrated significantly altered expression by Ras mutation or exposure to nicotine
Discussion
Lung cancer is the second most common cancer and the leading cause of death among all cancers. The overall prognosis for lung cancer has remained poor with a survival rate of mere 16% for the first 5 y With the advent of targeted molecular therapies this is expected to improve. In lieu of rapid development of resistance, it is imperative that we constantly add to the arsenal by developing novel targeted therapeutics. In this context, we have previously demonstrated the use of C. elegans as a simple model to study oncogenic mutants discovered in human lung cancer.36C. elegans engineered to over-express the MET mutant revealed an abnormal vulval phenotype with hyperplasia. Interestingly, exposure to nicotine significantly aggravated the above phenotype suggesting that C. elegans can be used as an in vivo model for rapid screening of c-MET mutants as well as drugs. Here we carried out systematic studies to evaluate the effects of chronic exposure to nicotine on wt and mutant worms such as vab-1 (Eph), SD551 (KRAS), RB2088 (MET) and 3 sli-1 (c-CBL) mutants, namely PS2728, PS1258 and MT13032. In general, the mutants suffered loss in survival, egg-laying capacity and fertility that was aggravated by exposure to nicotine. Locomotion studies revealed that with the increase in concentration of nicotine, there was a significant increase in locomotion speed in all strains except for vab-1. Wt N2 showed the maximum speed while SD551 had the least. We also found vab-1 mutant worms had increased circular path motion in that was enhanced with nicotine; an effect not observed in other mutant strains. Heat map analysis of gene expression profiling data clearly revealed up regulation of various kinases and phosphatases in C. elegans that are marginally expressed in N2 worms in response to chronic nicotine exposure. The expression of these genes was already elevated in SD551 that was further increased in response to nicotine. Our findings further strengthen the role of nicotine as a promoter of cancer.
C. elegans has served as an excellent model to investigate essential signaling pathways and behavioral responses due to its simple, non-redundant genetic makeup and ease of culture. Its behavioral responses to nicotine such as acute response, tolerance, withdrawal and sensitization mirror those that seen in mammals and require specific nAChRs that are highly conserved. For instance, lack of TRPC (transient receptor potential canonical) channels in C. elegans results in defective behavioral response to nicotine that can be rescued using human TRPC gene.14 Previously, we showed that C. elegans can serve as a model for mechanistic studies related to lung cancer. Worms forced to express a constitutively active MET mutant frequently associated with lung cancer suffered from significantly increased incidence of multi-vulval phenotype and associated hyperplasia that was exacerbated upon chronic exposure to nicotine.36 Here, we have extended the studies to investigate the behavioral and genetic changes in response to chronic nicotine treatment using select mutants of C. elegans that are relevant to human cancers.
The strong association between smoking and lung cancer is well known and has been the subject of intense investigation for more than 3 decades. The major addictive principle of cigarettes is the nicotine in the tobacco. Although, nicotine is not a carcinogen, it does play a significant role in promoting tumor growth and metastasis, and therefore can be termed a ‘cocarcinogen’. It promotes tumor growth through enhanced proliferation, cell motility and invasion, epithelial-mesenchymal transition, angiogenesis and by triggering signaling pathways that are associated with autocrine loops linked to tumor growth.51,52 Significant association between smoking and the development of pancreatic and lung cancers has been reported and moreover, the prognosis is poor in smokers suffering from above cancers.53,54 Cigarette smoke and the increased risk of pulmonary metastasis of breast cancer has been known for some time and was confirmed in a murine model.55,56 The link between nicotine and metastasis can be appreciated by the fact that it promotes epithelial mesenchymal transition, a process that is fundamental to cancer invasion.57 Interestingly, a dose dependent increase in the proliferation and invasion of breast, lung and pancreatic cancer cells using matrigel in response to nicotine was noted.3 It was also established by the same group that the effect was mediated through nAChRs.
Current experiments utilized relatively higher concentrations of nicotine that ranged from 50 to 500 μM. Since, little is known about the uptake mechanism of nicotine by the nematode C. elegans, it is rather difficult to determine the actual effective concentration of nicotine achieved in the worm. The range of concentrations used in the present study are comparable to those used by previously.58
We have utilized in this study several C. elegans mutants that have either non-functional or constitutive forms of key signaling molecules known to play a role in tumorigenesis. Others and we have previously shown that MET mutations, RAS mutations, CBL mutations can occur in the context of smoking. We have recently shown a critical role for EphB4 receptor tyrosine kinase in esophageal cancer and lung cancer.59C. elegans has one equivalent receptor vab-1. We used here the kinase inactive C. elegans mutant vab-1 (G912E) to investigate the chronic effects of nicotine.60 RAS is an important oncogene that is mutated in a number of tumors, such as pancreatic, colon and lung cancer. There are a number of KRAS mutations in lung cancers, especially on codon 12, 13 and 61. KRAS appears to be an oncogenic driver in lung cancer. Oncogenic mutations in the RAS gene are present in approximately 30% of all human cancers. KRAS mutations occur in more than 90% of pancreatic and colon cancers and about 20–30% in non-small-cell lung cancer. It is important to note that non-small lung cancer patients with KRAS mutations are also unresponsive to EGFR or ALK targeted therapies. In general, the prognosis is poor with KRAS mutations.1,2,61,62 We therefore investigated its equivalent in C. elegans the mutant SD551 (GA89), a gain of function temperature sensitive mutant that is highly active at 20°C but remains non-functional at 15°C.
We were one of the first to identify the RTK MET as an important target in lung and other cancers and our mechanistic, translational and clinical studies conducted over the past 20 y have now yielded several small molecule chemotherapeutic drugs that are now in various clinical trials.63 Also in this study, we utilized the non-functional MET receptor kinase ortholog RB2088 (F11E6.8) that has a 900 base pairs deletion in the kinase domain. Various RTKs that contribute to tumorigenesis are negatively regulated by c-CBL, an E3 ubiquitin ligase. We recently reported that c-CBL frequently suffers loss-of-function mutations in lung cancer and plays a key role in tumorigenesis.35 Here, we also utilized 3 (c-CBL ortholog) mutants: PS2728, PS1258 and MT13032, that are non-functional.
The mutants in general revealed lower egg-laying capacity and fertility compared to wt worms that were further worsened by chronic nicotine treatment. This is in agreement with the fact that cigarette smoking is associated with lower fecundity rates, adverse reproductive outcomes and higher risk of IVF failures; however it should be remembered that cigarette smoke has more than 1000 compounds of which the principal addictive compound is nicotine.64 Our gene expression profiling studies revealed that nicotine in general enhanced the expression of various kinases and phosphatases that are marginally expressed in wt worms and play a role in cell proliferation and tumor growth. This was however not apparent in SD551 mutant worm. One explanation could be due to the fact that the expression of both kinases and phosphatases in general and MAPK in particular were relatively high to start with as the mutation in question confers gain-of-function in mutants grown at RT. The above result is in accordance with the fact that constitutively activated RAS is oncogenic and plays a key role in tumorigenesis. Most importantly, the multi vulval phenotype seen in SD551 worms is exacerbated upon chronic treatment. In conclusion, our studies further strengthen our contention that the simple soil nematode C. elegans can be used to investigate some very basic aspects of cancer biology. It would now be useful to determine if this model can be used to test novel inhibitory strategies for various mutants and environmental exposure.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
This work is in part supported from NIH/NCI grant P30 CA014599 to University of Chicago Comprehensive Cancer Center, Cancer Research Foundation, Mesothelioma Association Research Foundation, Guy Geleerd Memorial and V Foundation to RS and by the Institute of Translational Medicine at the University of Chicago’s K12 award (NIH-NCI: K12CA139160) and the NIH-NIDDK P30 DK020595 to HKI.
References
- 1.De Biasi M, Dani JA.. Reward, addiction, withdrawal to nicotine. Annl Rev Neurosci 2011; 34:105–30; PMID:21438686; http://dx.doi.org/ 10.1146/annurev-neuro-061010-113734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Foll B., Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handbook Exp Pharmacol 2009; 335–67; PMID:19184655; http://dx.doi.org/ 10.1007/978-3-540-69248-5_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, Haura E, et al. . Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. International journal of cancer. J Int du Cancer 2009; 124:36–45; PMID:18844224; http://dx.doi.org/ 10.1002/ijc.23894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, Chellappan S. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PloS One 2009; 4:e7524; PMID:19841737; http://dx.doi.org/ 10.1371/journal.pone.0007524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 2003; 111:81–90; PMID:12511591; http://dx.doi.org/ 10.1172/JCI200316147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk A, Almedom RB, Schedletzky T, Anderson SD, Yates JR 3rd, Schafer WR. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J 2005; 24:2566–78; PMID:15990870; http://dx.doi.org/ 10.1038/sj.emboj.7600741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AK, Davis P, Hodgkin J, Sattelle DB. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Inver Neurosci: IN 2007; 7:129–31; PMID:17503100; http://dx.doi.org/ 10.1007/s10158-007-0049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popovici C, Roubin R, Coulier F, Pontarotti P, Birnbaum D. The family of Caenorhabditis elegans tyrosine kinase receptors: similarities and differences with mammalian receptors. Genome Res 1999; 9:1026–39; PMID:10568743; http://dx.doi.org/ 10.1101/gr.9.11.1026 [DOI] [PubMed] [Google Scholar]
- 9.Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci: Off J Soc Toxicol 2008; 106:5–28; PMID:18566021; http://dx.doi.org/ 10.1093/toxsci/kfn121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettinger JC, Leung K, Bolling MH, Goldsmith AD, Davies AG. Lipid environment modulates the development of acute tolerance to ethanol in Caenorhabditis elegans. PloS One 2012; 7:e35192; PMID:22574115; http://dx.doi.org/ 10.1371/journal.pone.0035192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro PV, Khare S, Young BD, Clarke SG. Caenorhabditis elegans battling starvation stress: low levels of ethanol prolong lifespan in L1 larvae. PloS One 2012; 7:e29984; PMID:22279556; http://dx.doi.org/ 10.1371/journal.pone.0029984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JR, Li Y, Rankin CH. Effects of developmental exposure to ethanol on Caenorhabditis elegans. Alcoholism, Clin Exp Res 2008; 32:853–67; PMID:18336629; http://dx.doi.org/ 10.1111/j.1530-0277.2008.00639.x [DOI] [PubMed] [Google Scholar]
- 13.Dillon J, Andrianakis I, Mould R, Ient B, Liu W, James C, O'Connor V, Holden-Dye L. Distinct molecular targets including SLO-1 and gap junctions are engaged across a continuum of ethanol concentrations in Caenorhabditis elegans. FASEB J: Off Pub Federat Am Soc Exp Biol 2013; 27:4266–78; PMID:23882127; http://dx.doi.org/ 10.1096/fj.11-189340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 2006; 127:621–33; PMID:17081982; http://dx.doi.org/ 10.1016/j.cell.2006.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green RM, Gally F, Keeney JG, Alper S, Gao B, Han M, Martin RJ, Weinberger AR, Case SR, Minor MN, et al. . Impact of cigarette smoke exposure on innate immunity: a Caenorhabditis elegans model. PloS One 2009; 4:e6860; PMID:19718433; http://dx.doi.org/ 10.1371/journal.pone.0006860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley AE. Worms clear the smoke surrounding nicotine addiction. Cell 2006; 127:460–2; PMID:17081968; http://dx.doi.org/ 10.1016/j.cell.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 17.Kwon JY, Hong M, Choi MS, Kang S, Duke K, Kim S, Lee S, Lee J. Ethanol-response genes and their regulation analyzed by a microarray and comparative genomic approach in the nematode Caenorhabditis elegans. Genomics 2004; 83:600–14; PMID:15028283; http://dx.doi.org/ 10.1016/j.ygeno.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 18.Lin CH, Sa S, Chand J, Rankin CH. Dynamic and persistent effects of ethanol exposure on development: an in vivo analysis during and after embryonic ethanol exposure in Caenorhabditis elegans. Alcoholism, Clin Exp Res 2013; 37Suppl 1:E190–8; PMID:22725623; http://dx.doi.org/ 10.1111/j.1530-0277.2012.01856.x [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Mould R, Dillon J, Glautier S, Andrianakis I, James C, Pugh A, Holden-Dye L, O'Connor V. A differential role for neuropeptides in acute and chronic adaptive responses to alcohol: behavioural and genetic analysis in Caenorhabditis elegans. PloS One 2010; 5:e10422; PMID:20454655; http://dx.doi.org/ 10.1371/journal.pone.0010422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieto-Fernandez F, Andrieux S, Idrees S, Bagnall C, Pryor SC, Sood R. The effect of opioids and their antagonists on the nocifensive response of Caenorhabditis elegans to noxious thermal stimuli. Inver Neurosci: IN 2009; 9:195–200; PMID:20397037; http://dx.doi.org/ 10.1007/s10158-010-0099-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellings L, Pereira S, Qian C, Dixon-McDougall T, Nowak C, Zhao B, Tyndale RF, van der Kooy D. Nicotine-motivated behavior in Caenorhabditis elegans requires the nicotinic acetylcholine receptor subunits acr-5 and acr-15. Euro J Neurosci 2013; 37:743–56; PMID:23351035; http://dx.doi.org/ 10.1111/ejn.12099 [DOI] [PubMed] [Google Scholar]
- 22.Sobkowiak R, Kowalski M, Lesicki A. Concentration- and time-dependent behavioral changes in Caenorhabditis elegans after exposure to nicotine. Pharmacol, Biochem, Behav 2011; 99:365–70; PMID:21624385; http://dx.doi.org/ 10.1016/j.pbb.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 23.Waggoner LE, Hardaker LA, Golik S, Schafer WR. Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics 2000; 154:1181–92; PMID:10757762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward A, Walker VJ, Feng Z, Xu XZ. Cocaine modulates locomotion behavior in C. elegans. PloS One 2009; 4:e5946; PMID:19536276; http://dx.doi.org/ 10.1371/journal.pone.0005946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest 2000; 105:3–7; PMID:10619854; http://dx.doi.org/ 10.1172/JCI9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengyel E, Sawada K, Salgia R. Tyrosine kinase mutations in human cancer. Curr Mol Med 2007; 7:77–84; PMID:17311534; http://dx.doi.org/ 10.2174/156652407779940486 [DOI] [PubMed] [Google Scholar]
- 27.Pisick E, Jagadeesh S, Salgia R. Receptor tyrosine kinases and inhibitors in lung cancer. Sci World J 2004; 4:589–604; PMID:15349502; http://dx.doi.org/ 10.1100/tsw.2004.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castano J, Davalos V, Schwartz S Jr, Arango D. EPH receptors in cancer. Histol Histopathol 2008; 23:1011–23; PMID:18498077 [DOI] [PubMed] [Google Scholar]
- 29.Catenacci DV, Cervantes G, Yala S, Nelson EA, El-Hashani E, Kanteti R, El Dinali M, Hasina R, Brägelmann J, Seiwert T, et al. . RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol, Ther 2011; 12:9–46; PMID:21543897; http://dx.doi.org/ 10.4161/cbt.12.1.15747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattler M, Salgia R. The MET axis as a therapeutic target. Update Cancer Therap 2009; 3:109–18; PMID:20368753; http://dx.doi.org/ 10.1016/j.uct.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine, Growth Fact Rev 2004; 15:419–33; PMID:15561600; http://dx.doi.org/ 10.1016/j.cytogfr.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 32.Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, Loganathan S, Kanteti R, Reichman T, Nallasura V, et al. . Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res 2008; 68:132–42; PMID:18172305; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanteti R, Nallasura V, Loganathan S, Tretiakova M, Kroll T, Krishnaswamy S, Faoro L, Cagle P, Husain AN, Vokes EE, et al. . PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest; J Tec Methods Pathol 2009; 89:301–14; PMID:19139719; http://dx.doi.org/ 10.1038/labinvest.2008.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki H, Hikosaka Y, Kawano O, Moriyama S, Yano M, Fujii Y. Evaluation of Kras gene mutation and copy number gain in non-small cell lung cancer. J Thorac Oncol: Off Pub Int Assoc Study Lung Cancer 2011; 6:15–20; PMID:21150464; http://dx.doi.org/ 10.1097/JTO.0b013e31820594f0 [DOI] [PubMed] [Google Scholar]
- 35.Tan YH, Krishnaswamy S, Nandi S, Kanteti R, Vora S, Onel K, Hasina R, Lo FY, El-Hashani E, Cervantes G, et al. . CBL is frequently altered in lung cancers: its relationship to mutations in MET and EGFR tyrosine kinases. PloS One 2010; 5:e8972; PMID:20126411; http://dx.doi.org/ 10.1371/journal.pone.0008972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui SS, Loganathan S, Krishnaswamy S, Faoro L, Jagadeeswaran R, Salgia R. C. elegans as a model organism for in vivo screening in cancer: effects of human c-Met in lung cancer affect C. elegans vulva phenotypes. Cancer Biol, Ther 2008; 7:856–63; PMID:18340114; http://dx.doi.org/ 10.4161/cbt.7.6.5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77:71–94; PMID:4366476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team , R: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. 2014. http://www.R-project.org/ [Google Scholar]
- 39.Therneau TM, GP. Modelling survival data: Extending the cox model. New York: Springer, 2000. [Google Scholar]
- 40.TM T. A package for survival analysis in S. R package version 2.37-7. 2014. http://cran.r-project.org/web/packages/survival. [Google Scholar]
- 41.Bull K, Cook A, Hopper NA, Harder A, Holden-Dye L, Walker RJ. Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int J Parasitol 2007; 37:627–36; PMID:17157854; http://dx.doi.org/ 10.1016/j.ijpara.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 42.Mendenhall AR, LeBlanc MG, Mohan DP, Padilla PA. Reduction in ovulation or male sex phenotype increases long-term anoxia survival in a daf-16-independent manner in Caenorhabditis elegans. Physiol Genomics 2009; 36:167–78; PMID:19050081; http://dx.doi.org/ 10.1152/physiolgenomics.90278.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzgerald V, Mensack M, Wolfe P, Thompson H. A transfer-less, multi-well liquid culture feeding system for screening small molecules that affect the longevity of Caenorhabditis elegans. Bio Techniques 2009; 47:ix-xv; PMID:20041852; http://dx.doi.org/ 10.2144/000113277 [DOI] [PubMed] [Google Scholar]
- 44.Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol 2001; 49:303–13; PMID:11745666; http://dx.doi.org/ 10.1002/neu.10014 [DOI] [PubMed] [Google Scholar]
- 45.Ramot D, Johnson BE, Berry TL Jr, Carnell L, Goodman MB. The parallel worm tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PloS One 2008; 3:e2208; PMID:18493300; http://dx.doi.org/ 10.1371/journal.pone.0002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47; PMID:25605792; http://dx.doi.org/ 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012; 28:882–3; PMID:22257669; http://dx.doi.org/ 10.1093/bioinformatics/bts034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 2003; 100:9440–5; PMID:12883005; http://dx.doi.org/ 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 2009; 4:44–57; PMID:19131956; http://dx.doi.org/ 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 50.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1–13; PMID:19033363; http://dx.doi.org/ 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke JP, Bitterman H. Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med 2004; 36:33–40; PMID:15000345; http://dx.doi.org/ 10.1080/07853890310017576 [DOI] [PubMed] [Google Scholar]
- 52.Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK, Rangnekar VM. Nicotinic modulation of therapeutic response in vitro and in vivo. International journal of cancer. J Int du Cancer 2012; 131:2519–27; PMID:22447412; http://dx.doi.org/ 10.1002/ijc.27556 [DOI] [PubMed] [Google Scholar]
- 53.Richardson GE, Tucker MA, Venzon DJ, Linnoila RI, Phelps R, Phares JC, Edison M, Ihde DC, Johnson BE. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Int Med 1993; 119:383–90; PMID:8393311; http://dx.doi.org/ 10.7326/0003-4819-119-5-199309010-00006 [DOI] [PubMed] [Google Scholar]
- 54.Vander Ark W, DiNardo LJ, Oliver DS. Factors affecting smoking cessation in patients with head and neck cancer. The Laryngoscope 1997; 107:888–92; PMID:9217125; http://dx.doi.org/ 10.1097/00005537-199707000-00010 [DOI] [PubMed] [Google Scholar]
- 55.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest 2001; 119:1635–40; PMID:11399684; http://dx.doi.org/ 10.1378/chest.119.6.1635 [DOI] [PubMed] [Google Scholar]
- 56.Murin S, Pinkerton KE, Hubbard NE, Erickson K. The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest 2004; 125:1467–71; PMID:15078760; http://dx.doi.org/ 10.1378/chest.125.4.1467 [DOI] [PubMed] [Google Scholar]
- 57.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 2005; 17:548–58; PMID:16098727; http://dx.doi.org/ 10.1016/j.ceb.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 58.Matta SG, Elberger AJ. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology 2007; 193:199–213; PMID:17404712; http://dx.doi.org/ 10.1007/s00213-007-0767-2 [DOI] [PubMed] [Google Scholar]
- 59.Hasina R, Mollberg N, Kawada I, Mutreja K, Kanade G, Yala S, Surati M, Liu R, Li X, Zhou Y, et al. . Critical role for the receptor tyrosine kinase EPHB4 in esophageal cancers. Cancer Res 2013; 73:184–94; PMID:23100466; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0915 [DOI] [PubMed] [Google Scholar]
- 60.Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes, Dev 2003; 17:187–200; PMID:12533508; http://dx.doi.org/ 10.1101/gad.1028303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S, et al. . The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Brit J Cancer 2005; 92:131–9; PMID:15597105; http://dx.doi.org/ 10.1038/sj.bjc.6602258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salgia R, Hensing T, Campbell N, Salama AK, Maitland M, Hoffman P, Villaflor V, Vokes EE. Personalized treatment of lung cancer. Semin Oncol 2011; 38:274–83; PMID:21421117; http://dx.doi.org/ 10.1053/j.seminoncol.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 63.Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol 2013; 31:1089–96; PMID:23401458; http://dx.doi.org/ 10.1200/JCO.2012.43.9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H. Effects of cigarette smoking on reproduction. Hum Reprod Update 2011; 17:76–95; PMID:20685716; http://dx.doi.org/ 10.1093/humupd/dmq033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.