Fig. 4. Mechanism of TRPV6 inactivation.
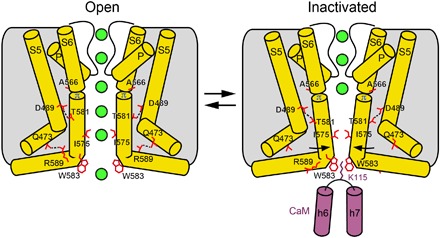
Cartoons represent the structural changes associated with CaM-induced inactivation of TRPV6. Only two of four TRPV6 subunits are shown, with the front and back subunits removed for clarity. The transition from the open to inactivated state involves tilting of the lower portions of the S6 helices toward the center of the pore at the alanine A566 gating hinge and closure of the pore stopping the permeation of ions (green spheres). The loss of the R589-Q473 salt bridges, which stabilize the α-to-π helical transition in S6 in the open state, is compensated by a cation-π interaction between K115 and the π-system of four tryptophans forming a cage at the pore intracellular entrance.
