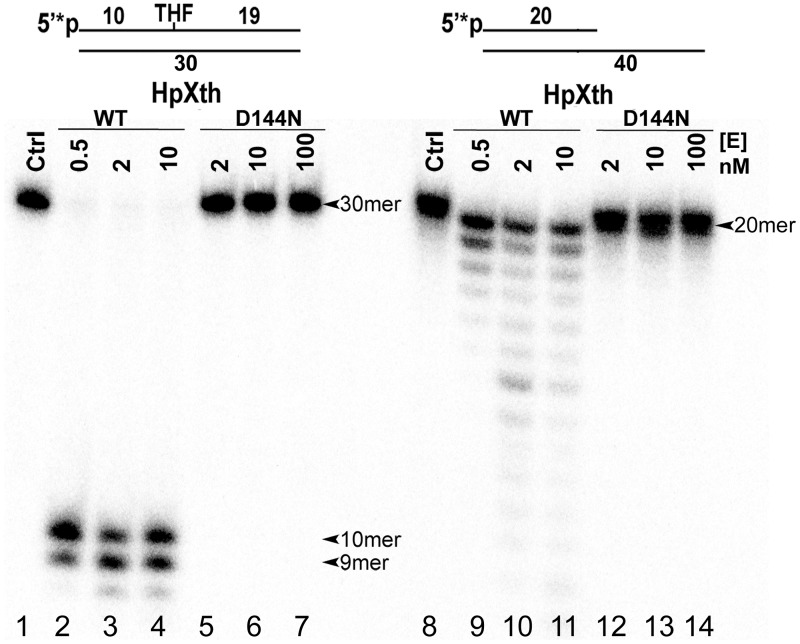
Fig 4. Comparative characterization of AP site cleavage and 3’→5’ exonuclease activities of wild-type HpXth and mutant HpXth-D144N.
In brief, 10 nM 5’-[32P]labeled THF•T and Exo20•RexTRec were incubated at 30 °C with increasing amounts of the HpXth-WT and HpXth-D144N proteins for 5 min at 30 °C in the standard reaction buffer. Lanes 1–7, THF•T; lane 1, control, no enzyme; lanes 2–4, 0.5, 2, and 10 nM HpXth-WT, respectively; lanes 5–7, 2, 10, and 100 nM HpXth-D144N, respectively; lanes 8–14, same as in lanes 1–7 but with Exo20•RexTRec. Products of the reaction were analyzed by denaturing PAGE. The arrows denote the position of the 30mer and 20mer substrates and 10mer and 9mer cleavage products. For details, see Materials and methods.