Key Points
Subsequent solid cancers after HCT occurred at younger ages than primary cancers and accounted for most common causes of deaths.
Subsequent solid cancers after HCT showed similar or worse survival compared with primary cancers in the general population.
Abstract
To characterize the outcomes of patients who developed a particular subsequent solid cancer after hematopoietic cell transplantation (HCT), age at cancer diagnosis, survival, and causes of death were compared with the respective primary cancer in the general population, using data from the national HCT registry and population-based cancer registries in Japan. Among 31 867 patients who underwent a first HCT between 1990 and 2013 and had progression-free survival at 1 year, 713 patients developed subsequent solid cancer. The median age at subsequent solid cancer diagnosis was 55 years, which was significantly younger than the 67 years for primary cancer patients in the general population (P < .001). The overall survival probability was 60% at 3 years after diagnosis of subsequent solid cancer and differed according to cancer type. Development of most solid cancers was associated with an increased risk of subsequent mortality after HCT. Subsequent solid cancers accounted for 76% of causes of death. Overall survival probabilities adjusted for age, sex, and year of diagnosis were lower in the HCT population than in the general population for colon, bone/soft tissue, and central nervous system cancers and did not differ statistically for other cancers. In conclusion, most subsequent solid cancers occurred at younger ages than primary cancers, emphasizing the need for cancer screening at younger ages. Subsequent solid cancers showed similar or worse survival compared with primary cancers. Biological and genetic differences between primary and subsequent solid cancers remain to be determined.
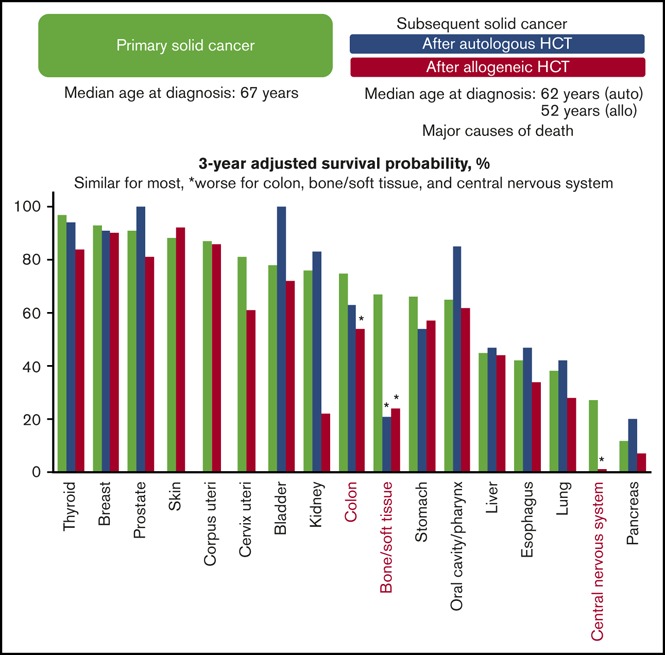
Visual Abstract
Introduction
Hematopoietic cell transplantation (HCT) is a curative treatment of a variety of hematologic diseases. Mortality associated with HCT has declined over the past several decades.1 As a result, the number of HCT survivors is growing,2 with a current estimate of one half million worldwide, and survivors are at considerable risk for many late effects.3 Development of subsequent solid cancer is one of the most debilitating late effects after HCT and accounts for 12% to 27% of deaths among long-term survivors.4-6 The cumulative incidence of subsequent solid cancer has been reported to be 1% to 6% at 10 years after HCT, and it continues to rise over time without a plateau.7-11 The most common sites include the oral cavity, skin, breast, and thyroid, and risks of solid cancer are elevated in the esophagus, liver, central nervous system, bone, and connective tissues in post-HCT patients compared with the general population.7-11 Notably, subsequent cancers in digestive organs such as the esophagus and colon are frequent in Japanese patients.10,12 Myeloablative total body irradiation (TBI), young age at HCT, chronic graft-versus-host disease (GVHD), and prolonged treatment with immunosuppressive medications are well-documented risk factors for the development of subsequent solid cancer.11
Although many studies have reported an increased incidence of subsequent solid cancer after HCT as well as associated risk factors, there are limited studies on the outcomes of patients who developed a specific subsequent solid cancer. One study examined the outcomes of 112 patients who developed subsequent solid cancer before 1995 after allogeneic HCT using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. The 5-year overall survival probabilities after the diagnosis of subsequent solid cancer varied by cancer type, being ∼90% for thyroid, testis, and melanoma; ∼50% for breast, oral cavity/pharynx, soft tissue, and female reproductive organs; and ≤20% for bone, lower gastrointestinal tract, and central nervous system.13 Although a formal statistical comparison was not performed, these probabilities seemed similar to those for primary cancers in the general population but were lower for female reproductive organs, bone, lower gastrointestinal tract, and central nervous system.
To characterize the outcomes of individual subsequent solid cancers after HCT in a contemporary cohort, we analyzed a large data set from the national HCT registry and population-based cancer registries in Japan. The aims of this study were to (1) elucidate overall survival rates and causes of death after the diagnosis of individual subsequent solid cancers, (2) examine the association between the development of different solid cancers and the risk of subsequent mortality after HCT, (3) examine factors associated with overall mortality, and (4) compare age at cancer diagnosis and survival between patients with subsequent cancer in the HCT population and those with primary cancer in the general population. The results of this study will provide physicians with important information for counseling and managing patients who develop subsequent solid cancers after HCT.
Patients and methods
Data source
HCT data were collected through the Transplant Registry Unified Management Program sponsored by the Japan Society for Hematopoietic Stem Cell Transplantation and the Japanese Data Center for Hematopoietic Cell Transplantation.14,15 More than 99% of all transplant centers in Japan reported and updated outcomes every year.14 Cancer survival data in the general population were collected from the population-based cancer registries through the Monitoring of Cancer Incidence in Japan project conducted by the Japan Cancer Surveillance Research Group.16 Registries that met the following criteria were considered high quality and were used for the study: (1) the proportion of patients for whom the death certificate provided the only notification to the registry was <25% or the proportion of patients for whom the death certificate provided the first notification to the registry was <30%, and (2) the mortality:incidence ratio was <0.67.16 The following 21 registries were included and encompassed 41% of the total Japanese population: Miyagi, Yamagata, Fukushima, Ibaraki, Tochigi, Gunma, Kanagawa, Fukui, Yamanashi, Aichi, Shiga, Osaka, Hyogo, Hiroshima, Kochi, Nagasaki, and Okinawa.16
The study cohort included all patients who underwent a first autologous or allogeneic HCT between 1990 and 2013 and whose progression-free survival was at least 1 year after HCT. Patients with Fanconi anemia, primary immunodeficiency diseases, or Down syndrome were excluded because of their inherent susceptibility to cancer. Patients with a history of solid cancer before HCT were also excluded. For comparison with primary cancer patients in the general population, patients from the population-based cancer registries who had the same age range and cancer type were used. This study was approved by the institutional review board of the National Cancer Center and was conducted in accordance with the Declaration of Helsinki.
Definitions
Cancer type was classified according to International Classification of Diseases, 10th revision. HLA matching for sibling and cord blood transplantation was assessed by serological data for the HLA-A, -B, and -DR loci. HLA matching for unrelated transplantation was assessed by using allele data for the HLA-A, -B, -C, and -DRB1 loci.15 HLA mismatch was defined in the GVHD vector when recipient antigens were not shared by the donor. The intensity of conditioning regimens was defined as described previously.17
Statistical analysis
Continuous variables were compared using the Mann-Whitney U test. The probability of overall survival was estimated by the Kaplan-Meier method from the diagnosis of solid cancer to death or last follow-up. Separate Cox models were used for autologous and allogeneic HCT to examine risk factors associated with overall mortality after the diagnosis of solid cancer. Factors with P < .05 in univariable testing were entered in a multivariable model. A backward stepwise procedure was used to develop a final model. A 2-sided P < .05 was considered statistically significant. Covariates included patient age at diagnosis of solid cancer, duration from HCT to diagnosis of solid cancer, patient sex, type of solid cancer, year of solid cancer diagnosis, primary disease requiring HCT, prior history of HCT, graft source, and the use of TBI in conditioning regimen. Donor relation, HLA matching, conditioning intensity, and a history of chronic GVHD were also included as covariates for patients who had allogeneic HCT. The proportional hazards assumption was tested for all variables considered in multivariable analysis, and no violations occurred. The 3-year survival was compared between the HCT and general population after adjustment for patient sex, patient age at cancer diagnosis, year of cancer diagnosis, and cancer type. The standardized mortality ratio (SMR) was calculated as the ratio of observed deaths to expected deaths in the age- and sex-matched general population of primary cancer, and 95% confidence intervals (CIs) were obtained using a Poisson regression model.18 All statistical analyses were performed using STATA version 12.1 (StataCorp, College Station, TX).
Results
Patient characteristics
Among 31 867 patients (10 678 autologous HCT and 21 189 allogeneic HCT) who underwent a first HCT and had progression-free survival at 1 year after HCT, 713 progression-free patients (2%) developed subsequent solid cancer. Solid cancer occurred after autologous HCT in 217 patients (30%) and after allogeneic HCT in 496 patients (70%). The median patient age at diagnosis of solid cancer was 55 years (range, 5-82 years). The median duration from HCT to solid cancer diagnosis was 5.9 years (range, 1.0-25 years). The duration from HCT to solid cancer diagnosis according to cancer type is shown in Figure 1. Four hundred forty-nine patients (63%) were male and 264 (37%) were female. The most frequent cancer was oropharyngeal cancer (n = 117), followed by cancers of the esophagus (n = 83), lung (n = 81), colon (n = 79), and stomach (n = 48). The most frequent primary disease was malignant lymphoma (n = 227), followed by acute myeloid leukemia (n = 171) and acute lymphoblastic leukemia (n = 101). Other characteristics are summarized in Table 1. When characteristics were compared between autologous and allogeneic HCT, patients after allogeneic HCT were younger at cancer diagnosis, had a longer duration from HCT to cancer diagnosis, more frequently had oropharyngeal or esophageal cancer, less frequently had colon or lung cancer, less frequently had prior HCT, more frequently had bone marrow transplantation using TBI-containing conditioning regimens for leukemia or myeloid neoplasms, and less frequently had lymphoma or plasma cell neoplasms.
Figure 1.
Duration from HCT to solid cancer diagnosis according to cancer type. Data are mean + standard error of the mean (SEM).
Table 1.
Characteristics of patients at diagnosis of subsequent solid cancer
| Characteristic | Total (N = 713), | Autologous (N = 217), | Allogeneic (N = 496), | P* |
|---|---|---|---|---|
| no. (%) | no. (%) | no. (%) | ||
| Median age at diagnosis of solid cancer (range), y | 55 (5-82) | 62 (10-82) | 52 (5-76) | <.001 |
| Median duration from HCT to diagnosis of solid cancer (range), y | 5.9 (1.0-25) | 4.9 (1.0-20) | 6.3 (1.0-25) | <.001 |
| Patient sex | .37 | |||
| Male | 449 (63) | 142 (65) | 307 (62) | |
| Female | 264 (37) | 75 (35) | 189 (38) | |
| Cancer type (ICD-10 code) | <.001 | |||
| Oral cavity/pharynx (C00-C14) | 117 (16) | 8 (4) | 109 (22) | |
| Esophagus (C15) | 83 (12) | 7 (3) | 76 (15) | |
| Stomach (C16) | 48 (7) | 20 (9) | 28 (6) | |
| Colon (C18-C20) | 79 (11) | 36 (17) | 43 (9) | |
| Liver (C22) | 20 (3) | 14 (6) | 6 (1) | |
| Pancreas (C25) | 25 (4) | 10 (5) | 15 (3) | |
| Lung (C33-C34) | 81 (11) | 38 (18) | 43 (9) | |
| Bone/soft tissue† (C40-C41) | 31 (4) | 9 (4) | 22 (4) | |
| Skin (C43-C44) | 28 (4) | 4 (2) | 24 (5) | |
| Breast (C50) | 38 (5) | 14 (6) | 24 (5) | |
| Cervix uteri (C53) | 9 (1) | 1 (<1) | 8 (2) | |
| Corpus uteri (C54) | 13 (2) | 4 (2) | 9 (2) | |
| Prostate (C61) | 22 (3) | 12 (6) | 10 (2) | |
| Kidney (C64-C65) | 15 (2) | 10 (5) | 5 (1) | |
| Bladder (C67) | 19 (3) | 7 (3) | 12 (2) | |
| Central nervous system (C71-72) | 16 (2) | 2 (1) | 14 (3) | |
| Thyroid (C73) | 28 (4) | 6 (3) | 22 (4) | |
| Other‡ | 41 (6) | 15 (7) | 26 (5) | |
| Year of diagnosis of solid cancer | .12 | |||
| 1993-1999 | 18 (3) | 9 (4) | 9 (2) | |
| 2000-2004 | 88 (12) | 31 (14) | 57 (11) | |
| 2005-2009 | 203 (28) | 52 (24) | 151 (30) | |
| 2010-2015 | 404 (57) | 125 (58) | 279 (56) | |
| Primary disease | <.001 | |||
| Acute myeloid leukemia | 171 (24) | 20 (9) | 151 (30) | |
| Acute lymphoblastic leukemia | 101 (14) | 7 (3) | 94 (19) | |
| Myelodysplastic syndrome/myeloproliferative neoplasms | 77 (11) | 1 (<1) | 76 (15) | |
| Chronic myelogenous leukemia | 60 (8) | 0 (0) | 60 (12) | |
| Malignant lymphoma | 227 (32) | 160 (74) | 67 (14) | |
| Plasma cell neoplasms | 34 (5) | 29 (13) | 5 (1) | |
| Adult T-cell leukemia/lymphoma | 20 (3) | 0 (0) | 20 (4) | |
| Aplastic anemia | 21 (3) | 0 (0) | 21 (4) | |
| Other | 2 (<1) | 0 (0) | 2 (<1) | |
| Prior history of HCT | 27 (4) | 13 (6) | 14 (3) | .04 |
| Graft source | <.001 | |||
| Bone marrow | 352 (49) | 12 (6) | 340 (69) | |
| Mobilized blood cells | 313 (44) | 205 (94) | 108 (22) | |
| Cord blood | 48 (7) | 0 (0) | 48 (10) | |
| TBI in conditioning regimen | 324 (45) | 21 (10) | 303 (61) | <.001 |
| Donor relation | ||||
| Related | 271 (55) | |||
| Unrelated | 225 (45) | |||
| HLA matching | ||||
| Match | 299 (60) | |||
| Mismatch | 143 (29) | |||
| Unknown | 54 (11) | |||
| Conditioning intensity | ||||
| Myeloablative | 245 (49) | |||
| Reduced intensity | 146 (29) | |||
| Unknown intensity | 105 (21) | |||
| Chronic GVHD | ||||
| None | 410 (58) | |||
| Limited | 110 (15) | |||
| Extensive | 193 (27) |
ICD-10, International Classification of Diseases, 10th revision.
P values for autologous vs allogeneic HCT.
Includes soft tissue cancers defined as ICD-O3 880-892, 912, and 918-926.
Nine patients had bile duct cancer, 8 cancer of unknown origin, 6 malignant peripheral nerve sheath tumor, 6 duodenum cancer, 4 ovarian cancer, 3 parotid gland cancer, 3 ureter cancer, 1 laryngeal cancer, and 1 submandibular gland cancer.
Survival and causes of death after diagnosis of subsequent solid cancer
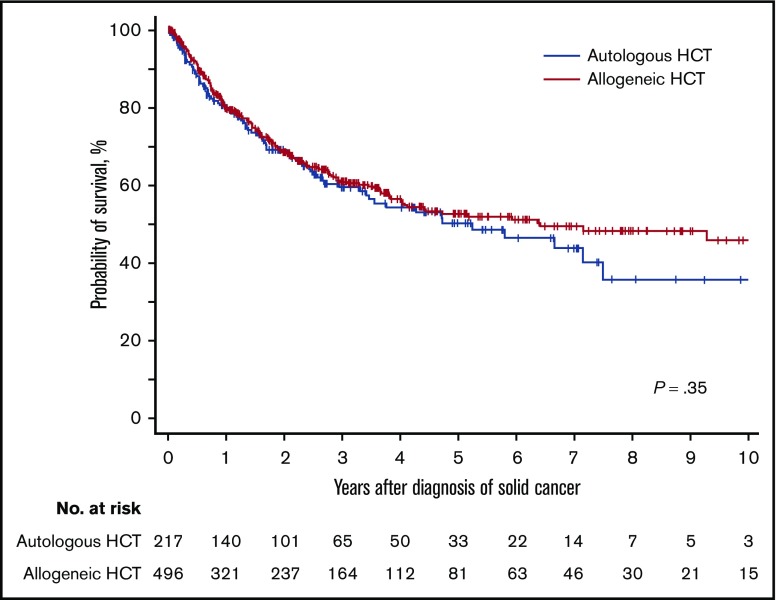
With a median follow-up duration of 2.6 years among survivors, the unadjusted overall survival probabilities at 3 years after the diagnosis of solid cancer were 60% (95% CI, 52% to 67%) for autologous HCT and 61% (95% CI, 56% to 66%) for allogeneic HCT and did not differ statistically between the two HCT types (P = .35; Figure 2). Multivariable Cox models for autologous HCT showed that female patients were associated with a lower risk of overall mortality (hazard ratio [HR], 0.59; 95% CI, 0.36-0.97; P = .039), and solid cancer diagnosis before 2000 was associated with a higher risk of overall mortality (HR, 2.45; 95% CI, 1.14-5.26; P = .022) (Table 2). Multivariable Cox models for allogeneic HCT showed 2 factors associated with overall mortality (Table 2). A longer duration from HCT to diagnosis of solid cancer was associated with a lower risk of overall mortality (HR, 0.94; 95% CI, 0.91-0.98; P = .005). Compared with oropharyngeal cancer, cancers of the esophagus, pancreas, lung, and central nervous system were associated with higher risks of overall mortality, while thyroid cancer was associated with a lower risk of overall mortality. Age at diagnosis of solid cancer, primary disease, graft source, the use of TBI in conditioning regimen, donor relation, HLA matching, conditioning intensity, and a history of chronic GVHD were not statistically associated with the risk of overall mortality.
Figure 2.
Probability of overall survival after diagnosis of subsequent solid cancer according to HCT type.
Table 2.
Multivariable analysis for factors associated with overall mortality after diagnosis of subsequent solid cancer
| Factor | Autologous (N = 217) | Allogeneic (N = 496) | ||||
|---|---|---|---|---|---|---|
| No. | Hazard ratio (95% CI) | P | No. | Hazard ratio (95% CI) | P | |
| Patient sex | ||||||
| Male | 142 | 1.00 (reference) | ||||
| Female | 75 | 0.59 (0.36-0.97) | .039 | |||
| Year of diagnosis of solid cancer | ||||||
| 1993-1999 | 9 | 2.45 (1.14-5.26) | .022 | |||
| 2000-2004 | 31 | 0.79 (0.42-1.49) | .47 | |||
| 2005-2009 | 52 | 0.80 (0.46-1.39) | .44 | |||
| 2010-2015 | 125 | 1.00 (reference) | ||||
| Duration from transplantation to cancer diagnosis (per year) | 496 | 0.94 (0.91-0.98) | .005 | |||
| Cancer type | ||||||
| Oral cavity/pharynx | 109 | 1.00 (reference) | ||||
| Esophagus | 76 | 2.43 (1.52-3.90) | <.001 | |||
| Stomach | 28 | 1.06 (0.48-2.34) | .89 | |||
| Colon | 43 | 1.35 (0.74-2.45) | .32 | |||
| Liver | 6 | 3.01 (0.92-9.90) | .069 | |||
| Pancreas | 15 | 6.78 (3.29-14.0) | <.001 | |||
| Lung | 43 | 2.54 (1.45-4.46) | .001 | |||
| Bone/soft tissue | 22 | 1.82 (0.91-3.65) | .093 | |||
| Skin | 24 | 0.69 (0.27-1.78) | .44 | |||
| Breast | 24 | 0.14 (0.02-1.05) | .056 | |||
| Cervix uteri | 8 | 0.84 (0.20-3.55) | .82 | |||
| Corpus uteri | 9 | 0.43 (0.06-3.13) | .40 | |||
| Prostate | 10 | 0.69 (0.16-2.89) | .61 | |||
| Kidney | 5 | 0.92 (0.12-6.71) | .93 | |||
| Bladder | 12 | 0.63 (0.15-2.63) | .52 | |||
| Central nervous system | 14 | 4.12 (2.01-8.45) | <.001 | |||
| Thyroid | 22 | 0.12 (0.02-0.87) | .036 | |||
| Others | 26 | 2.21 (1.13-4.33) | .021 | |||
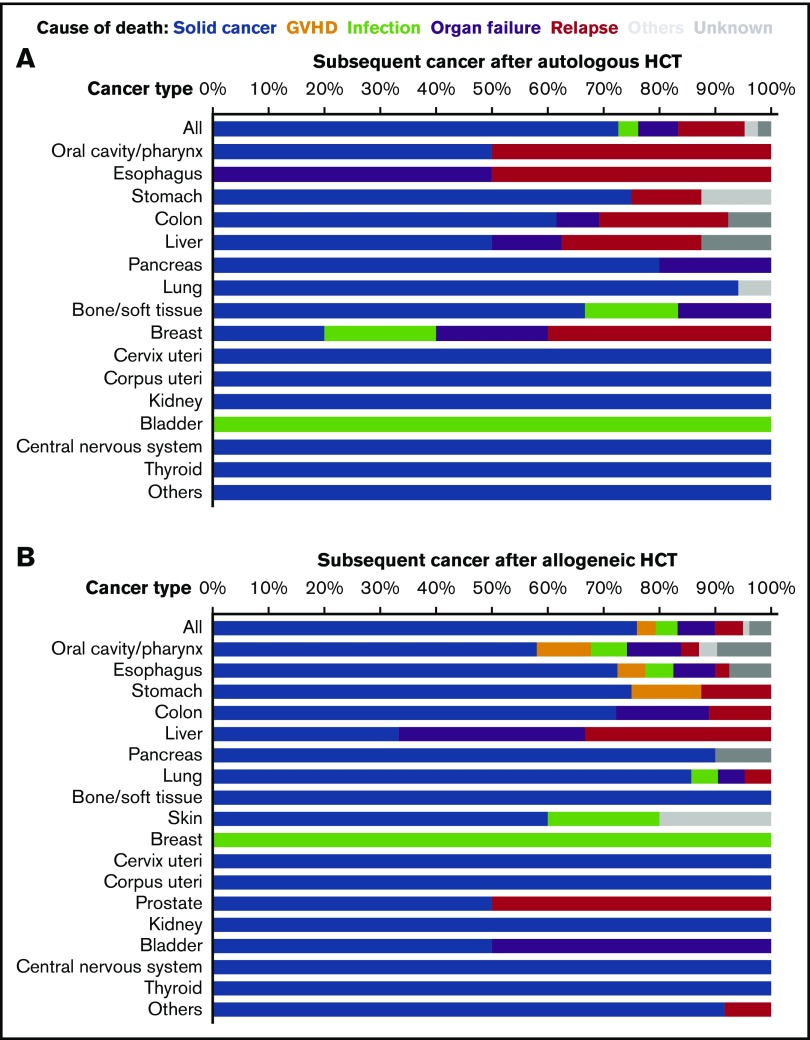
Regarding causes of death, subsequent solid cancer accounted for 73% of causes of death in patients who had autologous HCT and 76% of causes of death in those who had allogeneic HCT (Figure 3). Analysis in individual cancers showed that subsequent solid cancer was the most common cause of death, except for cancers of the esophagus, breast, and bladder after autologous HCT (Figure 3A), and except for liver and breast cancers after allogeneic HCT (Figure 3B).
Figure 3.
Causes of death among patients who developed subsequent solid cancer after HCT. (A) Autologous HCT. Skin and prostate cancers are excluded because no death occurred with these cancers. (B) Allogeneic HCT.
Association of subsequent solid cancer development with risk of subsequent overall mortality in HCT patients
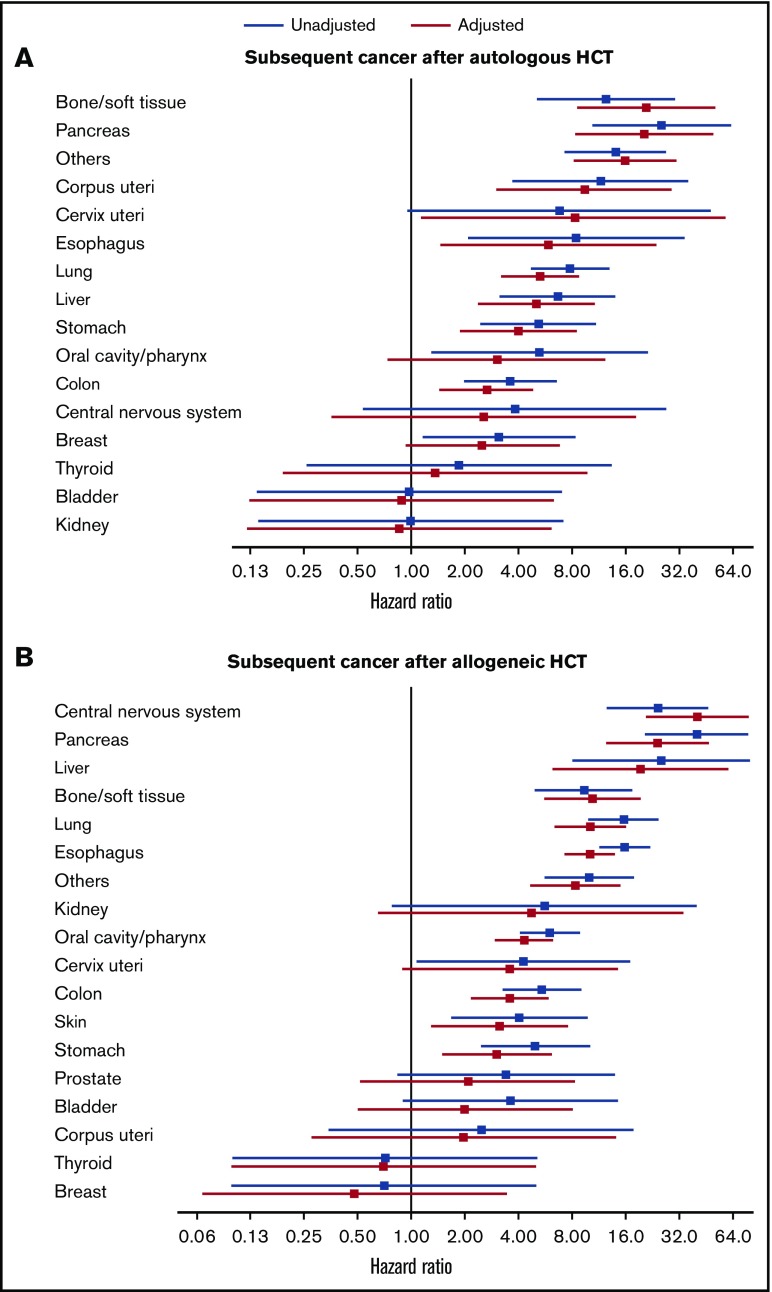
Time-varying Cox models were used to examine the association of solid cancer development with risk of subsequent overall mortality in the 31 867 patients with 1-year progression-free survival. Results are shown with unadjusted models and with models adjusted for factors associated with the risk of subsequent solid cancer development in prior studies (ie, patient age at HCT, prior chronic GVHD, and the use of TBI in conditioning regimen).10 Development of any solid cancer was associated with an increased risk of subsequent overall mortality after autologous HCT (adjusted HR, 3.81; 95% CI, 3.05-4.75; P < .001) and after allogeneic HCT (adjusted HR, 5.63; 95% CI, 4.81-6.59; P < .001). Analyses for individual cancers are shown in Figure 4. Compared with patients who did not develop subsequent solid cancer, those who developed solid cancer had an increased risk of subsequent overall mortality, except for those with cancers of the oral cavity/pharynx, central nervous system, breast, thyroid, bladder, and kidney after autologous HCT and except for those with cancers of the kidney, uterus, prostate, bladder, thyroid, and breast after allogeneic HCT.
Figure 4.
Association of individual solid cancer development with risk of subsequent overall mortality. Diagnosis of individual solid cancer was treated as time-varying mutually exclusive events. Patients who did not develop subsequent solid cancer were used as reference. Hazard ratios and 95% confidence intervals are shown with forest plots in unadjusted (blue) and adjusted (red) models. (A) Autologous HCT. Adjusted factors include patient age at transplantation and the use of TBI. Skin and prostate cancers are excluded because no deaths occurred with these cancers. (B) Allogeneic HCT. Adjusted factors include patient age at transplantation, prior chronic GVHD, and the use of TBI.
Comparison of age at diagnosis among patients with primary vs subsequent solid cancer
The ages at diagnosis of cancers were compared between patients with subsequent solid cancer after HCT and those with primary cancer in the general population with the same age range and cancer type by using data from the population-based cancer registries (Table 3). The median age at diagnosis of all solid cancers combined was significantly younger in the HCT population than in the general population (62 vs 67 years after autologous HCT, P < .001; 52 vs 67 years after allogeneic HCT, P < .001). This observation held true for lung and bladder cancers after autologous HCT and for cancers except for breast, uterus, prostate, and bladder after allogeneic HCT. The age at cancer diagnosis was younger after allogeneic HCT than after autologous HCT for cancers of the oral cavity/pharynx, esophagus, colon, liver, lung, skin, and thyroid.
Table 3.
Comparison of age at diagnosis between primary cancer in the general population and subsequent solid cancer after HCT
| Cancer type | No. | Median age at diagnosis (range) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| General | Autologous | Allogeneic | General | Autologous | Allogeneic | Autologous vs general | Allogeneic vs general | Autologous vs allogeneic | |
| All | 541 134 | 217 | 496 | 67 (5-82) | 62 (10-82) | 52 (5-76) | <.001 | <.001 | <.001 |
| Oral cavity/pharynx | 7 904 | 8 | 109 | 61 (17-71) | 62 (55-71) | 49 (17-68) | .50 | <.001 | <.001 |
| Esophagus | 13 804 | 7 | 76 | 65 (25-76) | 63 (56-71) | 57 (24-76) | .35 | <.001 | .03 |
| Stomach | 82 076 | 20 | 28 | 66 (19-77) | 62 (34-77) | 57 (18-71) | .055 | <.001 | .09 |
| Colon | 72 270 | 36 | 43 | 65 (17-75) | 62 (34-75) | 57 (17-71) | .08 | <.001 | .02 |
| Liver | 32 078 | 14 | 6 | 70 (14-82) | 67 (31-82) | 43 (12-61) | .06 | <.001 | .011 |
| Pancreas | 11 329 | 10 | 15 | 64 (29-72) | 66 (46-72) | 59 (29-69) | .51 | .01 | .08 |
| Lung | 63 833 | 38 | 43 | 69 (22-80) | 65 (46-80) | 59 (22-71) | .014 | <.001 | <.001 |
| Bone/soft tissue | 2 404 | 9 | 22 | 48 (9-63) | 41 (10-62) | 18 (9-63) | .10 | <.001 | .08 |
| Skin | 5 582 | 4 | 24 | 65 (20-75) | 67 (36-75) | 45 (20-64) | .85 | <.001 | .045 |
| Breast | 50 155 | 14 | 24 | 55 (29-71) | 57 (29-67) | 52 (31-71) | .44 | .32 | .19 |
| Cervix uteri | 6 459 | 1 | 8 | 44 (28-60) | 51 (NA) | 43 (28-60) | .49 | .66 | .44 |
| Corpus uteri | 7 298 | 4 | 9 | 56 (32-66) | 56 (52-66) | 54 (32-64) | .52 | .73 | .40 |
| Prostate | 18 481 | 12 | 10 | 65 (53-69) | 63 (54-69) | 63 (53-68) | .52 | .18 | .55 |
| Kidney | 9 051 | 10 | 5 | 60 (13-72) | 57 (42-72) | 51 (10-68) | .43 | .039 | .14 |
| Bladder | 6 959 | 7 | 12 | 62 (30-69) | 54 (33-66) | 62 (30-69) | .015 | .65 | .25 |
| Central nervous system | 750 | 2 | 14 | 43 (7-57) | 36 (14-57) | 13 (7-28) | .99 | <.001 | .20 |
| Thyroid | 9 507 | 6 | 22 | 57 (8-75) | 61 (20-68) | 24 (7-75) | .76 | <.001 | .009 |
General refers to general population.
Comparison of adjusted survival probabilities between patients with primary vs subsequent solid cancer
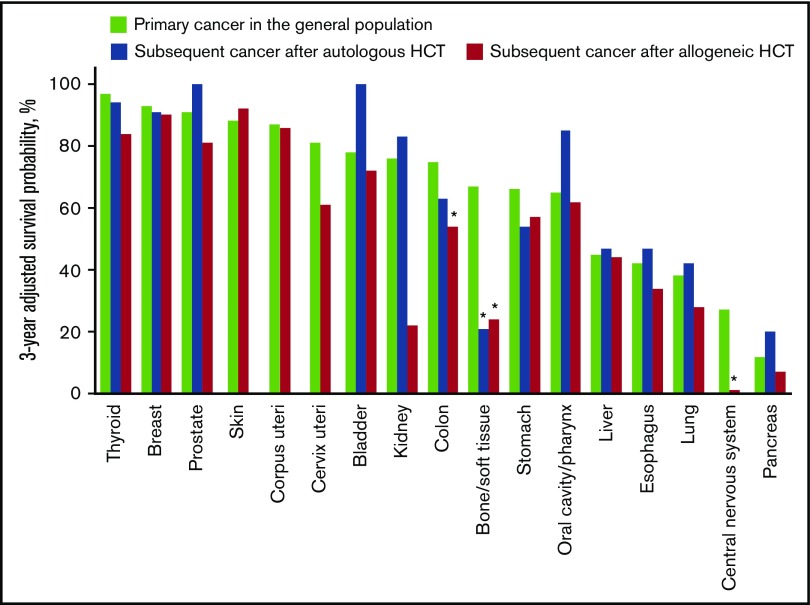
Overall survival probabilities adjusted for age, sex, and year of diagnosis were compared between patients with subsequent solid cancer after HCT and those with primary cancer in the general population, using the same data from the population-based cancer registries (Table 4). The adjusted overall survival probability was lower in the HCT population than in the general population in all cancers combined after allogeneic HCT (60% vs 72% at 3 years; HR, 1.41; 95% CI, 1.20-1.65; P < .001). Results for individual cancers are shown in Table 4 and Figure 5. The adjusted overall survival probabilities were lower in the HCT population than in the general population for bone/soft tissue cancer after autologous HCT (21% vs 67% at 3 years; HR, 2.93; 95% CI, 1.21-7.07; P = .017); and for colon cancer (54% vs 75% at 3 years; HR, 2.14; 95% CI, 1.27-3.62; P = .004), bone/soft tissue cancer (24% vs 67% at 3 years; HR, 3.16; 95% CI, 1.68-5.93; P < .001), and central nervous system cancer after allogeneic HCT (1% vs 27% at 3 years; HR, 4.54; 95% CI, 2.29-9.01; P < .001). The adjusted overall survival probabilities did not differ statistically between primary and subsequent solid cancers for other cancers.
Table 4.
Comparison of adjusted overall survival probabilities with primary cancer patients in the general population
| Cancer type | No. | 3-y adjusted overall survival* | Autologous vs general | Allogeneic vs general | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| General | Autologous | Allogeneic | General | Autologous | Allogeneic | HR* (95% CI) | P | HR* (95% CI) | P | |
| All | 541 134 | 217 | 496 | 0.72 | 0.65 | 0.60 | 1.21 (0.96-1.53) | .11 | 1.41 (1.20-1.65) | <.001 |
| Oral cavity/pharynx | 7 904 | 8 | 109 | 0.65 | 0.85 | 0.62 | 0.55 (0.08-3.93) | .56 | 0.99 (0.65-1.49) | .95 |
| Esophagus | 13 804 | 7 | 76 | 0.42 | 0.47 | 0.34 | 0.61 (0.15-2.44) | .48 | 1.15 (0.83-1.60) | .40 |
| Stomach | 82 076 | 20 | 28 | 0.66 | 0.54 | 0.57 | 1.61 (0.76-3.37) | .21 | 1.37 (0.65-2.87) | .41 |
| Colon | 72 270 | 36 | 43 | 0.75 | 0.63 | 0.54 | 1.57 (0.85-2.92) | .15 | 2.14 (1.27-3.62) | .004 |
| Liver | 32 078 | 14 | 6 | 0.45 | 0.47 | 0.44 | 0.95 (0.43-2.12) | .91 | 1.32 (0.42-4.10) | .63 |
| Pancreas | 11 329 | 10 | 15 | 0.12 | 0.20 | 0.07 | 0.81 (0.34-1.95) | .64 | 1.24 (0.67-2.31) | .49 |
| Lung | 63 833 | 38 | 43 | 0.38 | 0.42 | 0.28 | 0.88 (0.55-1.42) | .60 | 1.12 (0.73-1.72) | .61 |
| Bone/soft tissue | 2 404 | 9 | 22 | 0.67 | 0.21 | 0.24 | 2.93 (1.21-7.07) | .017 | 3.16 (1.68-5.93) | <.001 |
| Skin | 5 582 | 4 | 24 | 0.88 | NA | 0.92 | NA | NA | 0.60 (0.08-4.27) | .61 |
| Breast | 50 155 | 14 | 24 | 0.93 | 0.91 | 0.90 | 1.35 (0.19-9.57) | .77 | 0.99 (0.14-7.07) | 1.0 |
| Cervix uteri | 6 459 | 1 | 8 | 0.81 | NA | 0.61 | NA | NA | 2.71 (0.67-10.9) | .16 |
| Corpus uteri | 7 298 | 4 | 9 | 0.87 | NA | 0.86 | NA | NA | 1.59 (0.22-11.5) | .65 |
| Prostate | 18 481 | 12 | 10 | 0.91 | 1.00 | 0.81 | NA | NA | 3.26 (0.46-23.2) | .24 |
| Kidney | 9 051 | 10 | 5 | 0.76 | 0.83 | 0.22 | 0.63 (0.09-4.51) | .65 | 2.27 (0.32-16.1) | .41 |
| Bladder | 6 959 | 7 | 12 | 0.78 | 1.00 | 0.72 | NA | NA | 0.74 (0.10-5.27) | .76 |
| Central nervous system | 750 | 2 | 14 | 0.27 | NA | 0.01 | NA | NA | 4.54 (2.29-9.01) | <.001 |
| Thyroid | 9 507 | 6 | 22 | 0.97 | 0.94 | 0.84 | 2.01 (0.27-14.8) | .49 | 4.47 (0.63-31.9) | .14 |
NA, not applicable.
Adjusted for patient sex, patient age at cancer diagnosis, year of cancer diagnosis, and cancer type. Hazard ratios and P values were derived from multivariable Cox models.
Figure 5.
Age-, sex-, and year of diagnosis–adjusted overall survival probability at 3 years after diagnosis of subsequent solid cancer in the HCT population and primary cancer in the general population. Cancers in the skin, uterus, and central nervous system after autologous HCT are excluded because of the small number of patients. *P < .05.
Compared with the age- and sex-matched general population of primary cancers (Table 5), SMRs of subsequent solid cancers were elevated after both autologous and allogeneic HCT in all cancers combined (1.79; 95% CI, 1.43-2.21; P < .001; 1.66; 95% CI, 1.43-1.93; P < .001, respectively), colon cancer (2.20; 95% CI, 1.17-3.77; P = .02; 2.12; 95% CI, 1.26-3.35; P = .007, respectively), and bone/soft tissue cancer (3.16; 95% CI, 1.16-6.87; P = .03; and 2.97; 95% CI, 1.48-5.32; P = .003, respectively). The SMR after autologous HCT was elevated for breast cancer (5.00; 95% CI, 1.62-11.7; P = .01).
Table 5.
Expected and observed deaths according to cancer type
| Cancer type | Autologous | Allogeneic | ||||||
|---|---|---|---|---|---|---|---|---|
| Expected* | Observed | SMR (95% CI) | P | Expected* | Observed | SMR (95% CI) | P | |
| All | 47.0 | 84 | 1.79 (1.43-2.21) | <.001 | 107.6 | 179 | 1.66 (1.43-1.93) | <.001 |
| Oral cavity/pharynx | 2.3 | 2 | 0.87 (0.11-3.14) | 1.0 | 24.7 | 31 | 1.26 (0.85-1.78) | .25 |
| Esophagus | 1.8 | 2 | 1.11 (0.13-4.01) | 1.0 | 38.8 | 40 | 1.03 (0.74-1.40) | .89 |
| Stomach | 3.6 | 8 | 2.22 (0.96-4.38) | .16 | 5.7 | 8 | 1.40 (0.61-2.77) | .43 |
| Colon | 5.9 | 13 | 2.20 (1.17-3.77) | .02 | 8.5 | 18 | 2.12 (1.26-3.35) | .007 |
| Liver | 7.1 | 8 | 1.13 (0.49-2.22) | .83 | 3.4 | 3 | 0.88 (0.18-2.58) | 1.0 |
| Pancreas | 5.0 | 5 | 1.00 (0.32-2.33) | 1.0 | 9.8 | 10 | 1.02 (0.49-1.88) | 1.0 |
| Lung | 18.7 | 17 | 0.91 (0.53-1.46) | .81 | 18.8 | 21 | 1.12 (0.69-1.71) | .67 |
| Bone/soft tissue | 1.9 | 6 | 3.16 (1.16-6.87) | .03 | 3.7 | 11 | 2.97 (1.48-5.32) | .003 |
| Skin | NA | 0 | NA | NA | 2.7 | 5 | 1.85 (0.60-4.32) | .27 |
| Breast | 1.0 | 5 | 5.00 (1.62-11.7) | .01 | 1.5 | 1 | 0.67 (0.02-3.71) | 1.0 |
| Cervix uteri | NA | 1 | NA | NA | 0.7 | 2 | 2.86 (0.35-10.3) | .31 |
| Corpus uteri | NA | 3 | NA | NA | 0.6 | 1 | 1.67 (0.04-9.29) | 1.0 |
| Prostate | 0.6 | 0 | NA | NA | 0.6 | 2 | 3.33 (0.40-12.0) | .24 |
| Kidney | 1.5 | 1 | 0.67 (0.02-3.71) | 1.0 | 0.5 | 1 | 2.00 (0.05-11.1) | .79 |
| Bladder | 1.2 | 1 | 0.83 (0.02-4.64) | 1.0 | 1.2 | 2 | 1.67 (0.20-6.02) | .68 |
| Central nervous system | NA | 1 | NA | NA | 7.1 | 10 | 1.41 (0.68-2.59) | .35 |
| Thyroid | 0.2 | 1 | 5.00 (0.13-27.9) | .36 | 0.4 | 1 | 2.50 (0.06-13.9) | .66 |
Expected number of deaths in the age- and sex-matched general population of primary cancer.
Discussion
This registry study elucidated different overall survival probabilities according to types of subsequent solid cancers after HCT. Importantly, development of most solid cancers was associated with an increased risk of subsequent mortality, and solid cancer was the most common cause of death, with few exceptions. This study also demonstrated that many subsequent solid cancers after HCT occurred at younger ages and were associated with comparable or worse survival compared with the same type of primary cancers in the general population, emphasizing the need for cancer screening at younger ages in HCT survivors.
It was shown that subsequent cancers of the skin and colon that developed after organ transplantation occurred at a younger age than primary cancer in the general population.19-21 A plausible explanation for the early carcinogenesis after organ transplantation is that immunosuppressive conditions can impair the cancer surveillance mechanism.22 Immunosuppressive conditions may also increase the risk of viral reactivation and virus-related malignancies.23 Chronic mucosal inflammation caused by chronic GVHD may increase the risk of carcinogenesis after allogeneic HCT. In fact, cancer development at a younger age was particularly evident after allogeneic HCT. Thus, cumulative chemotherapy-induced DNA damage, prolonged immunosuppressive conditions, and chronic mucosal inflammation, particularly after allogeneic HCT, could account for the younger age of cancer development after HCT.
Previous studies have reported 3-year overall survival probabilities of 50% to 60% after diagnosis of subsequent solid cancer,13,24,25 and the probability was similar in this study. In analyses of individual cancers, adjusted overall survival probabilities were favorable for thyroid, breast, prostate, and skin cancers, whereas they were poor for bone/soft tissue, central nervous system, and pancreatic cancers. These results were consistent with those reported in the study of the CIBMTR registry.13
Adjusted overall survival probabilities were lower in patients with subsequent cancer compared with those with primary cancer in the general population for colon, central nervous system, and bone/soft tissue cancers after allogeneic HCT. The CIBMTR registry study also reported worse survival probabilities in subsequent cancers of the lower gastrointestinal tract, central nervous system, and bones and joints after allogeneic HCT.13 Although these results require careful interpretation because of the lack of information on cancer stage at diagnosis and treatment details in both registries, similarities between the 2 independent registry studies might indicate the presence of biological differences between primary and subsequent cancers. A recent population-based study showed that subsequent cancers of the breast, thyroid, soft tissues, colon, central nervous system, and cervix were associated with worse survival than primary cancers.26 Many subsequent cancers that occurred after organ transplantation were characterized by aggressive behavior.27 Several studies showed that subsequent colorectal cancer after organ transplantation had a more aggressive phenotype and worse prognosis compared with primary cancer in the general population, possibly because of immunosuppression.19,20,28 Several population-based studies also showed that cancer histology differed between subsequent and primary cancer, and that the risk of death was higher with subsequent cancer despite increased surveillance, screening, and access to care among previous cancer patients.29,30 Additional studies are necessary to elucidate biological and genetic differences between primary cancers and subsequent cancers after HCT.
Multivariable analysis showed that female patients and cancer diagnosis before 2000 were associated with overall mortality after autologous HCT, and a longer duration from HCT to diagnosis of solid cancer was associated with a lower risk of overall mortality after allogeneic HCT. Patient age at diagnosis of solid cancer, type of HCT, primary disease, conditioning intensity, and the use of TBI in conditioning regimen were not statistically associated with the risk of overall mortality. Female cancer patients showed higher survival probabilities than male cancer patients in the study of population-based cancer registries.16 Because patients are likely to recover physically and because mortality rates decrease with time after HCT, it would not be surprising if later development of solid cancer were associated with lower mortality. The CIBMTR study, however, did not show a decrease in mortality associated with longer duration between HCT and subsequent cancer diagnosis after allogeneic HCT.13
This study had several strengths and limitations. We used a national HCT registry and population-based cancer databases in Japan, and thus the results could be generalized to all HCT survivors nationwide. Conversely, the major drawback of the current HCT registry database was the lack of information on subsequent cancer stage at diagnosis, treatment details, health behaviors, or cytogenetic features. Collection of these details is warranted in future studies to determine whether adverse outcomes were related to advanced stage as a result of late diagnosis, intolerability to standard treatment, aggressive behaviors, or combinations of these factors. Finally, the results for some cancers were based on a small number of cases and should be interpreted with caution.
This registry study clearly showed that subsequent solid cancer was associated with an increased risk of mortality and accounted for the major cause of death in most cancers. Outcomes differed largely according to cancer type. These results should prove useful when counseling and managing patients who develop subsequent solid cancers after HCT. Because many of these cancers occurred at a younger age than primary cancers in the general population, our results highlight the importance of cancer screening at younger ages among HCT survivors and will help to make evidence-based prevention and screening guidelines for HCT survivors. Additional studies are warranted to characterize the biological and genetic differences of subsequent solid cancers after HCT.
Acknowledgment
This work was supported by grant 18K08345 from the Japan Society for the Promotion of Science (Y.I.).
Authorship
Contribution: Y.I. designed the study; T. Matsuda, N.D., K.I., T. Mori, S.T., H.Y., A.K., H. Nakamae, T.S., H.H., J.S., H.A., and T.F. collected data; K.T., S.K., H. Nakasone, H. Nishimori, S.Y., T.I., and T.Y. wrote the manuscript; Y.I., and Y.A. performed the statistical analysis; and all authors interpreted data, critically revised the manuscript for important intellectual content, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group appears in “Appendix.”
Correspondence: Yoshihiro Inamoto, Department of Hematopoietic Stem Cell Transplantation, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: yinamoto@ncc.go.jp.
Appendix: study group members
The members of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group are: Y.I., Y.A., Minako Iida, Takayuki Ishikawa, Keiichi Isoyama, Masami Inoue, Kumi Oshima, Shinichiro Okamoto, S.K., A.K., Rika Sakai, Masaaki Shiohara, Shuichi Taniguchi, K.T., H. Nakasone, Makoto Hirokawa, Shin Fujisawa, Yasuo Horikoshi, Masato Masuda, Hidetsugu Mihara, Yuki Asano-Mori, T.Y., Yasushi Ishida, Aika Seto, Nahoko Hatsumi, Akira Hayakawa, Atsushi Sato, H. Nishimori, Masako Toyosaki, S.Y., Koichi Miyamura, Chikako Kiyotani, Raine Tatara, Toshihiro Matsukawa, and Satoshi Yoshioka.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. . Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Pasquini MC, Aljurf M, et al. ; Worldwide Network for Blood and Marrow Transplantation (WBMT). One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2(3):e91-e100. [DOI] [PubMed] [Google Scholar]
- 3.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102(4):614-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Francisco L, Carter A, et al. . Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Counts GW Jr, Appelbaum FR, et al. . Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atsuta Y, Hirakawa A, Nakasone H, et al. ; Late Effect and Quality of Life Working Group of the Japan Society for Hematopoietic Cell Transplantation. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1702-1709. [DOI] [PubMed] [Google Scholar]
- 7.Curtis RE, Rowlings PA, Deeg HJ, et al. . Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897-904. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JD, Curtis RE, Socié G, et al. . Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia S, Louie AD, Bhatia R, et al. . Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464-471. [DOI] [PubMed] [Google Scholar]
- 10.Atsuta Y, Suzuki R, Yamashita T, et al. ; Japan Society for Hematopoietic Cell Transplantation. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25(2):435-441. [DOI] [PubMed] [Google Scholar]
- 11.Inamoto Y, Shah NN, Savani BN, et al. . Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(8):1013-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Kurosawa S, Tajima K, et al. . Increased incidence of oral and gastrointestinal secondary cancer after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(5):789-791. [DOI] [PubMed] [Google Scholar]
- 13.Ehrhardt MJ, Brazauskas R, He W, Rizzo JD, Shaw BE. Survival of patients who develop solid tumors following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(1):83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103(1):3-10. [DOI] [PubMed] [Google Scholar]
- 15.Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103(1):11-19. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol. 2011;41(1):40-51. [DOI] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, et al. . Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddell FD. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health. 1984;38(1):85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papaconstantinou HT, Sklow B, Hanaway MJ, et al. . Characteristics and survival patterns of solid organ transplant patients developing de novo colon and rectal cancer. Dis Colon Rectum. 2004;47(11):1898-1903. [DOI] [PubMed] [Google Scholar]
- 20.Johnson EE, Leverson GE, Pirsch JD, Heise CP. A 30-year analysis of colorectal adenocarcinoma in transplant recipients and proposal for altered screening. J Gastrointest Surg. 2007;11(3):272-279. [DOI] [PubMed] [Google Scholar]
- 21.Mullen DL, Silverberg SG, Penn I, Hammond WS. Squamous cell carcinoma of the skin and lip in renal homograft recipients. Cancer. 1976;37(2):729-734. [DOI] [PubMed] [Google Scholar]
- 22.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77(12):1777-1782. [DOI] [PubMed] [Google Scholar]
- 23.Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer. 2009;125(8):1755-1763. [DOI] [PubMed] [Google Scholar]
- 24.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21(7):1352-1358. [DOI] [PubMed] [Google Scholar]
- 25.Shimada K, Yokozawa T, Atsuta Y, et al. . Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36(2):115-121. [DOI] [PubMed] [Google Scholar]
- 26.Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017;3(11):1554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campistol JM, Cuervas-Mons V, Manito N, et al. ; ATOS Working Group. New concepts and best practices for management of pre- and post-transplantation cancer. Transplant Rev (Orlando). 2012;26(4):261-279. [DOI] [PubMed] [Google Scholar]
- 28.Pollard JD, Hanasono MM, Mikulec AA, Le QT, Terris DJ. Head and neck cancer in cardiothoracic transplant recipients. Laryngoscope. 2000;110(8):1257-1261. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb M, Freyer DR. Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer. 2014;120(8):1155-1161. [DOI] [PubMed] [Google Scholar]
- 30.Sadler C, Goldfarb M. Comparison of primary and secondary breast cancers in adolescents and young adults. Cancer. 2015;121(8):1295-1302. [DOI] [PubMed] [Google Scholar]