ABSTRACT
Body weight (BW) and rearing photoperiod are important factors affecting sexual maturation rate and reproductive performance in broiler breeders. The current experiment used a 2 × 3 factorial arrangement of treatments to study the interaction between BW and rearing photoperiod on reproductive performance in group housed broiler breeder hens, while minimizing variation in BW. Hens (n = 180) were fed with a precision feeding system to allocate feed individually to achieve the breeder-recommended target curve (Standard) or to a target curve that reached the 21 wk BW at 18 wk (High). Hens were on 8L:16D, 10L:14D, or 12L:12D photoschedules during rearing and were photostimulated at 21 wk with a 16L:8D photoschedule. Sexual maturity (defined as age at first egg) and individual egg production to 55 wk were recorded. At 55 wk, proportional weights of individual body components were determined by dissection. Differences were reported as significant at P ≤ 0.05. A significant interaction between BW and rearing photoschedule affected age at sexual maturity and egg production. In the High BW treatment, age at sexual maturity did not differ between hens under the 8L:16D and 10L:14D photoschedules (173 vs. 172 d, respectively). In the Standard BW treatment, the 12L:12D rearing photoperiod delayed sexual maturity compared with the 8L:16D rearing photoperiod (266 vs. 180 d, respectively). All hens on the High BW treatment laid at least 1 egg before the end of the experiment. Conversely, 3.3, 18.1, and 37.6% of Standard BW hens on the 8L:16D, 10L:14D, and 12L:12D photoschedules, respectively, never commenced egg production. At the end of the experiment, proportional breast weight was higher and proportional fatpad weight was lower in Standard compared to High BW hens (25.8 vs. 27.5% and 2.4 vs. 1.5% of BW, respectively). We conclude that increased BW partially counters the effect of longer photoschedules on sexual maturity in broiler breeders and that dissipation of the photorefractory state depends on BW.
Keywords: daylength, egg production, reproduction, photorefractoriness, sexual maturity
INTRODUCTION
In broiler breeders, rearing photoperiod and BW both affect sexual maturation and productivity, and to date those effects have been reported as independent. Photoperiod needs to be controlled during rearing to dissipate juvenile photorefractoriness. Under natural conditions, juvenile photorefractoriness occurs when pullets are exposed to long photoperiods (≥13 h), which prevents birds from becoming sexually mature in the same year in which they are hatched, thus avoiding offspring in suboptimal conditions (Lewis, 2006). In broiler breeders, rearing photoperiod determines the dissipation rate of the photorefractory state and the age at sexual maturation (Payne, 1975; Lewis et al., 2004). Exposure to short rearing photoperiods (≤10 h) accelerates dissipation of the photorefractory state and synchronizes onset of lay after photostimulation. However, under long rearing photoperiods (≥13 h), sexual maturity is delayed and egg production is reduced (Lewis et al., 2003; Lewis, 2006).
Independent of photoperiod, higher than recommended BW at the end of rearing accelerated sexual maturity (age at first egg), whereas a lower than recommended BW delayed sexual maturity (Fattori et al., 1991; Renema et al., 2001a, b; Hocking, 2004; Ekmay et al., 2012). However, other studies did not find the same result (Zuidhof et al., 2007; van Emous et al., 2013). Target BW curves of the latter studies converged at peak production, whereas target BW curves in the former were not aligned during the laying period (van Emous et al., 2013). Therefore, both target BW and the timing and level of feed restriction may affect sexual maturity and reproductive efficiency.
Earlier studies reported effects of rearing photoperiod as independent of BW (Gous and Cherry, 2004; Lewis et al., 2004, 2005). However, over the past decades, variation in BW in group housed flocks has increased as a result of increased levels of feed restriction and feed competition (Renema et al., 2007). Previous studies investigating the interaction between rearing photoperiod and BW were performed on hens reared in groups. In these studies, high within-treatment BW variation may have overshadowed interactions between BW and photoperiod treatments.
Therefore, the aim of the current research was to investigate the interaction between BW and rearing photoperiod on group housed broiler breeder reproductive performance with minimal BW variation. It was hypothesized that onset of lay would be delayed in lower BW hens under extended photoperiods, and egg production would be reduced. Conversely, within photoschedule treatments, higher BW hens would dissipate photorefractoriness and mature more quickly, thereby increasing total egg production.
MATERIALS AND METHODS
Experimental Design
The animal protocol for the study was approved by the University of Alberta Animal Care and Use Committee for Livestock and followed principles established by the Canadian Council on Animal Care Guidelines and Policies (CCAC, 2009). The experiment was conducted as a 2 × 3 factorial arrangement of treatments with pullets reared either on a breeder-recommended target BW curve (Standard; Aviagen, 2016) or an accelerated target BW curve reaching the 21 wk BW at 18 wk (High), and maintained under 8L:16D, 10L:14D, or 12L:12D photoschedules during rearing. The resulting High target BW was 22% higher than the Standard target BW at 21 wk of age.
Animals and Housing
Ross 708 broiler breeder chicks (n = 180; provided by Aviagen, Huntsville, AL) were neck tagged for individual identification, and randomly allocated in 6 environmentally controlled rooms measuring 3.8 × 2.2 m (30 chicks per room). Floors of the rooms were covered with wood shavings at an approximate depth of 5 cm. Each room was equipped with a precision feeding (PF) system (Zuidhof et al., 2016, 2017), which controlled individual feed intake to achieve and adhere to the assigned target BW curves. Water was provided ad libitum with nipple drinkers during the entire experiment and a fountain style supplemental drinker was provided in each pen during the first week. From day 0 to 16, birds were trained to use the PF system and were fed ad libitum. At day 16, birds were tagged with a radio frequency identification wing band, and randomly assigned to either the Standard or High BW treatment, such that approximately half of the birds per room were assigned to either target BW curve. From day 16 onwards all birds were fed individually and were allowed access to feed for a duration of 45 s when their BW, measured in real-time by the PF system, was lower than their treatment target BW. When their measured BW was equal to or higher than their treatment target, birds were ejected from the PF system. Treatment BW targets were updated on an hourly basis. At the start of the experiment, pairs of rooms were randomly assigned to either an 8L:16D, 10L:14D, or 12L:12D rearing photoschedule. For the first 2 d, a 23L:1D photoschedule was used to ensure full access to water and feed, after which the photoperiod was decreased by 2 h/d until the treatment photoschedule was reached. Hens from all treatments were photostimulated at week 21 with a single abrupt step to 16L:8D. The light source (60% red, 20% green, and 20% blue LED light bulbs; PGR-11, AgriLux, Cambridge, ON) provided 8 lux during rearing and 25 lux during the laying phase. For the first 3 wk, chicks received a standard wheat based starter diet (2900 AME, 19% CP, 1.1% Ca); from week 4 to 23 pullets received a wheat and barley based grower diet (2589 AME, 14.2% CP, and 0.9% Ca); from week 23 to 34 hens received a wheat based peak layer diet (2689 AME, 15.0% CP, and 3.3% Ca); and from week 35 to 55 hens received a wheat based post peak layer diet (2682 AME 14.6% CP, and 3.3% Ca).
At week 18, a nest box with 8 nesting sites equipped with radio frequency identification readers was installed in each room, which identified eggs of individual hens. At week 21, 3 roosters were introduced to each room. Roosters had been reared in a separate location under an 8L:16D photoschedule and precision fed on their breeder-recommended target BW curve (Aviagen, 2016).
Data Collection
For the first 2 wk, pullets were weighed manually on a daily basis to confirm growth and use of the PF system. Birds that were not growing were trained individually to use the PF system. After individual feeding started, the PF system recorded individual BW and feed intake on a per visit basis. Feed intake and visit frequency was checked on a daily basis to ensure all birds were accessing the PF system. Because it would not be possible for floor eggs to be linked with individual hens because hens on different BW treatments were housed in the same room, cloaca of all hens were palpated daily to detect hard-shelled eggs in the shell gland to measure age at first egg and individual egg production from 20 to 36 wk. This ensured a precise estimate of age at first egg for each individual bird. As the majority of the birds on the 8L:16D photoschedule treatment had entered lay by week 36, from 36 wk onward, daily palpation was performed every second week. Eggs assigned to individual hens were weighed daily. Eggs between 40 and 90 g were included in statistical analysis for egg weight. Eggs weighing more than 90 g were considered double yolked eggs and were analyzed separately. Mortality (including cull) was recorded throughout the experiment. At week 55, all remaining hens were killed by cervical dislocation and dissected. Abdominal fat pad, filled gastrointestinal tract (GIT), breast muscle (total weight of pectoralis major and pectoralis minor), heart, liver, oviduct (without content), and ovary weight were recorded. The GIT consisted of the complete digestive tract including pancreas, from 2 cm anterior to the crop up to but not including the bursa, with fat adhering to the proventriculus and gizzard removed (included in abdominal fatpad weight). In addition, the number of large yellow follicles (LYF) was recorded.
Statistical Analysis
All ANOVA were conducted using the MIXED procedure of SAS (Version 9.4. SAS Institute Inc., Cary, NC, 2012). Pairwise differences between means were determined with the PDIFF option of the LSMEANS statement and were considered significant at P ≤ 0.05. Tukey's range test was used to compare treatment means. Hen was the experimental unit, except for cumulative hens in lay and percentage of hens that did not commence egg production before week 55. For the latter, hens within each BW treatment within each chamber were randomly assigned to 1 of 3 groups, after which the parameters were calculated per group and group was used as experimental unit. The model used for the coefficient of variation for BW (BW CV), egg production, cumulative hens in lay, and egg weight data included BW treatment, rearing photoschedule, and age as fixed effects and all 2 and 3-way interactions. Random variation due to hen was accounted for in all serial measurements. As a result of insufficient data points early in lay, egg weight was analyzed from week 30 onward. The model used for age at first egg, BW at age at first egg, percentage of hens that did not commence egg production before week 55, cumulative egg production, and cumulative feed intake (CFI) data included BW treatment and rearing photoschedule as fixed effects, and their interaction. The model used for the dissection data included BW treatment and rearing photoschedule as fixed effects, and their interaction and a binary random variable indicating whether the hen had laid her first egg.
RESULTS AND DISCUSSION
BW, BW Variation, and Feed Intake
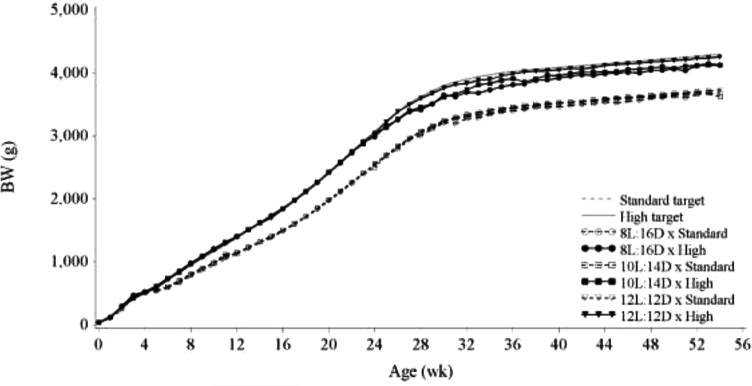
Actual Standard and High BW profiles closely matched their target profiles up to 24 wk of age (Figure 1). BW CV throughout the experiment (Table 1) was dependent on age (P = 0.015) and on BW treatment (P = 0.003). As no significant pairwise differences were indicated after Tukey's range test, Table 1 shows results of the least significant difference test. BW CV of the High BW treatment increased after photostimulation compared to the Standard BW treatment. We hypothesize that this was mainly because hens on the High BW treatment started laying earlier compared to hens on the Standard BW treatment and sexually matured at a BW below their target BW (Table 3; Figure 1). These hens remained at a BW lower than their target throughout the study, suggesting that they reached their mature BW. As their mature BW was lower than the target, BW variability increased in the High BW treatment. By using the PF system, the BW CV in our experiment was well below the 8 to 15% reported in recent studies on the effects of photoperiod or BW on broiler breeder performance (Gous and Cherry, 2004; van Emous et al., 2013; Zuidhof et al., 2015), and lower than reported BW CV in a previous study using a PF system (Zuidhof et al., 2017).
Figure 1.
BW of hens fed to achieve either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule.
Table 1.
Coefficient of variation for BW (BW CV) at various ages of hens fed with a precision feeding system to achieve a High or Standard BW1 curve and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule (RPS).
| BW CV (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | RPS | Week 4 | SEM | Week 7 | SEM | Week 14 | SEM | Week 21 | SEM | Week 27 | SEM | Week 40 | SEM | Week 54 | SEM | |
| Week2 | 3.6w | 0.6 | 2.5w,x | 0.6 | 0.7z | 0.6 | 0.8y,z | 0.6 | 1.7x-z | 0.6 | 2.4w-y | 0.6 | 2.7w,x | 0.6 | ||
| BW × week | High | 4.3 | 0.7 | 2.3 | 0.7 | 0.9 | 0.7 | 0.5 | 0.7 | 2.7a | 0.7 | 3.6a | 0.7 | 3.4 | 0.7 | |
| Standard | 2.8 | 0.7 | 2.8 | 0.7 | 0.5 | 0.7 | 1.1 | 0.7 | 0.8b | 0.7 | 1.2b | 0.7 | 2.0 | 0.7 | ||
| RPS × week | 8L:16D | 4.2 | 1.0 | 2.7 | 1.0 | 0.3 | 1.0 | 1.0 | 1.0 | 2.7 | 1.0 | 4.0 | 1.0 | 3.3 | 1.0 | |
| 10L:14D | 1.9 | 1.0 | 2.9 | 1.0 | 0.4 | 1.0 | 0.3 | 1.0 | 1.6 | 1.0 | 2.2a | 1.0 | 3.7 | 1.0 | ||
| 12L:12D | 4.6 | 1.0 | 2.0 | 1.0 | 1.3 | 1.0 | 1.1 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| BW × RPS × week | High | 8L:16D | 4.9a,b | 1.2 | 2.5 | 1.2 | 0.2 | 1.2 | 0.8 | 1.2 | 4.1a | 1.2 | 5.4a | 1.2 | 4.6a | 1.2 |
| 10L:14D | 2.2b | 1.2 | 3.1 | 1.2 | 0.4 | 1.2 | 0.3 | 1.2 | 2.6a,b | 1.2 | 4.0a,b | 1.2 | 4.0a | 1.2 | ||
| 12L:12D | 5.9a | 1.2 | 1.3 | 1.2 | 2.2 | 1.2 | 0.3 | 1.2 | 1.3a,b | 1.2 | 1.4b,c | 1.2 | 1.4a,b | 1.2 | ||
| Standard | 8L:16D | 3.6a,b | 1.2 | 2.8 | 1.2 | 0.4 | 1.2 | 1.2 | 1.2 | 1.2b | 1.2 | 2.6b,c | 1.2 | 2.0a,b | 1.2 | |
| 10L:14D | 1.5b | 1.2 | 2.7 | 1.2 | 0.5 | 1.2 | 0.3 | 1.2 | 0.6b | 1.2 | 0.5c | 1.2 | 3.4a,b | 1.2 | ||
| 12L:12D | 3.3a,b | 1.2 | 2.8 | 1.2 | 0.5 | 1.2 | 1.8 | 1.2 | 0.5b | 1.2 | 0.7b,c | 1.2 | 0.6b | 1.2 | ||
a–cLSMeans within a column and treatment group lacking a common superscript differ (P ≤ 0.05).
w–zLSMeans within a row and treatment group lacking a common superscript differ (P ≤ 0.05).
1Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
2 P-values for the different sources of variation were as follows: week, P = 0.015; BW, P = 0.003; RPS, P = 0.214; BW × RPS, P = 0.584; week × BW, P = 0.056; week × RPS, P = 0.377; week × BW × RPS, P = 0.714.
Table 3.
Age at first egg (AFE)1, percentage of hens that did not commence production before 55 wk (not laid), BW at AFE1, cumulative egg production (eggs), and overall egg weight of hens fed to achieve a High or Standard BW2 curve and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule (RPS).
| BW | RPS | AFE (d) | SEM | Not laid (%) | SEM | BW at AFE (g) | SEM | Eggs | SEM | Egg weight (g) | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | High | 185b | 5 | 0.0b | 2.07 | 3,278a | 33 | 138a | 6 | 64.8 | 0.1 | |
| Standard | 219a | 5 | 19.7a | 2.07 | 3,057b | 37 | 88b | 6 | 64.4 | 0.2 | ||
| RPS | 8L:16D | 177b | 5 | 1.7b | 2.53 | 2,935b | 38 | 138a | 7 | 65.2a | 0.1 | |
| 10L:14D | 192b | 6 | 9.0b | 2.53 | 3,053b | 45 | 120a | 8 | 63.4b | 0.1 | ||
| 12L:12D | 238a | 6 | 18.8a | 2.53 | 3,513a | 45 | 81b | 7 | 65.1a | 0.1 | ||
| BW × RPS | High | 8L:16D | 173c | 8 | 0.0c | 3.58 | 3,137b | 53 | 152 | 10 | 64.8a,b | 0.2 |
| 10L:14D | 172c | 9 | 0.0c | 3.58 | 3,074b | 63 | 151 | 12 | 64.2b | 0.3 | ||
| 12L:12D | 210b | 8 | 0.0c | 3.58 | 3,621a | 55 | 111 | 10 | 65.3a | 0.2 | ||
| Standard | 8L:16D | 180b,c | 8 | 3.3b,c | 3.58 | 2,732c | 54 | 125 | 10 | 65.6a | 0.2 | |
| 10L:14D | 212b | 9 | 18.1b | 3.58 | 3,033b | 65 | 89 | 11 | 62.7c | 0.3 | ||
| 12L:12D | 266a | 10 | 37.6a | 3.58 | 3,405a | 71 | 51 | 10 | 64.9a,b | 0.3 | ||
| Source of variation | –––––––––––––––––––––––––––––––- P-value ------––––––––––––––––––––––––––– | |||||||||||
| BW | <0.001 | <0.001 | <0.001 | <0.001 | 0.087 | |||||||
| RPS | <0.001 | <0.001 | <0.001 | < 0.001 | <0.001 | |||||||
| BW × RPS | 0.012 | <0.001 | 0.010 | 0.160 | <0.001 | |||||||
a–cLSMeans within a column and treatment group lacking a common superscript differ (P ≤ 0.05).
1Hens that did not commence egg production before week 55 were excluded from the analysis.
2 Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
For CFI, there was a significant interaction between BW and rearing photoschedule. During the rearing phase, CFI was lower for hens on the Standard BW treatment compared to hens on the High BW treatment (Table 2). This was anticipated as lower energy and protein requirements are expected for growth and maintenance for hens fed to a lower BW target. Within the High BW treatment, CFI was lower in hens on the 8L:16D photoschedule compared to hens on the 10L:14D and 12L:12D photoschedule. Gous and Cherry (2004) also showed an interaction between rearing photoperiod and BW target on CFI, but a clear explanation for the result was not evident. In their study, CFI was 110 and 770 g higher in the 8L:16D treatment compared to the 17L:7D treatment for the 1,550 and 2,500 g 21 wk target; however, for the 2,150 and 2,850 g 21 wk target, CFI was 160 and 360 g lower in the shorter photoperiod treatment. This is not consistent with the current results; however, their long photoperiod was 5 h longer than in the current study. We hypothesize that increased photoperiod beyond 8L:16D increased the period of activity of the pullets during the 24 h period, which might have increased the energetic expenditure for locomotion. We recognize that further investigation into energy expenditure and energy allocation is needed. During the laying phase, CFI of hens on the High BW target did not differ, whereas CFI of hens on the Standard BW target was reduced in the 10L:14D and 12L:12D rearing photoschedules compared to the 8L:16D rearing photoschedule. This latter interaction was likely the result of differences in egg production, and respective increase in ME intake to support egg production (Romero et al., 2009). Mortality throughout the trial did not differ significantly between treatment groups.
Table 2.
Cumulative feed intake (CFI) of broiler breeder hens from day 16 to week 21 (rearing phase) and from week 21 to 55 (laying phase), fed to achieve a High or Standard BW1 curve and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule (RPS).
| Rearing phase | Laying phase | |||||
|---|---|---|---|---|---|---|
| BW | RPS | CFI (g) | SEM | CFI (g) | SEM | |
| BW | High | 9,063a | 36 | 33,718a | 572 | |
| Standard | 7,337b | 35 | 26,286b | 561 | ||
| RPS | 8L:16D | 8,091b | 41 | 32,324a | 651 | |
| 10L:14D | 8,260a | 46 | 30,505a | 731 | ||
| 12L:12D | 8,249a | 44 | 27,177b | 698 | ||
| BW × RPS | High | 8L:16D | 8,865b | 58 | 34,652a | 920 |
| 10L:14D | 9,157a | 67 | 34,939a | 1069 | ||
| 12L:12D | 9,166a | 61 | 31,563a,b | 976 | ||
| Standard | 8L:16D | 7,316c | 58 | 29,996b | 920 | |
| 10L:14D | 7,364c | 62 | 26,072c | 997 | ||
| 12L:12D | 7,331c | 62 | 22,791c | 997 | ||
| Source of variation | –-–––––––––––– P-value ––––––––––––– | |||||
| BW | <0.001 | <0.001 | ||||
| RPS | 0.008 | <0.001 | ||||
| BW × RPS | 0.008 | 0.044 | ||||
a–cLSMeans within a column and treatment group lacking a common superscript differ (P ≤ 0.05).
1Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
Sexual Maturity and Egg Production
In line with previous findings, increased BW accelerated sexual maturity (Table 3). Hens on the High BW treatment started laying 34 d earlier than hens on the Standard BW treatment. The advance of sexual maturity was 1.6, 9.1, and 12.7 d per 100 g increase in BW at 20 wk of age for the 8L:16D, 10L:14D, and 12L:12D rearing photoschedule, respectively. For the 8L:16D rearing photoschedule, similar observations were previously reported, where sexual maturity advanced between 1.5 and 3.0 d per 100 g increase in BW at 20 wk of age (Renema et al., 2001a; Gous and Cherry, 2004; Sun and Coon, 2005). Conversely, Fattori et al. (1991) showed that a decrease in BW of 100 g at 20 wk delayed sexual maturity by 7.3 d. The latter suggests that there is a minimum BW threshold that needs to be met for sexual maturation to proceed. However, this threshold might depend on rearing photoperiod, as there was an interaction between rearing photoperiod and BW treatment on BW at sexual maturity (Table 3).
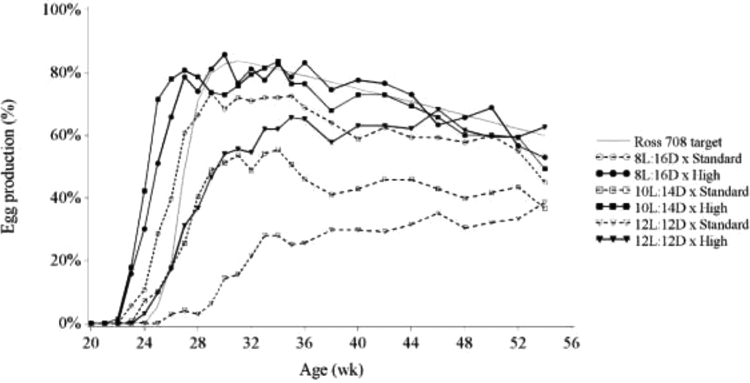
Sexual maturity was delayed and egg production reduced in hens reared on increased photoperiods (Table 3; Figure 2), similar to the results of Lewis et al. (2003). Hens reared on 10L:14D tended to start laying later than hens on the 8L:16D photoschedule (15 d, P = 0.082) and hens reared on the 12L:12D photoschedule started laying 61 d later (P < 0.001) than hens on the 8L:16D photoschedule. This confirms that modern broiler breeders are photorefractory at hatch, and that the photorefractory state was dissipated by a short photoperiod in a photoperiod dependent manner.
Figure 2.
Hen day egg production of hens fed to achieve either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule.
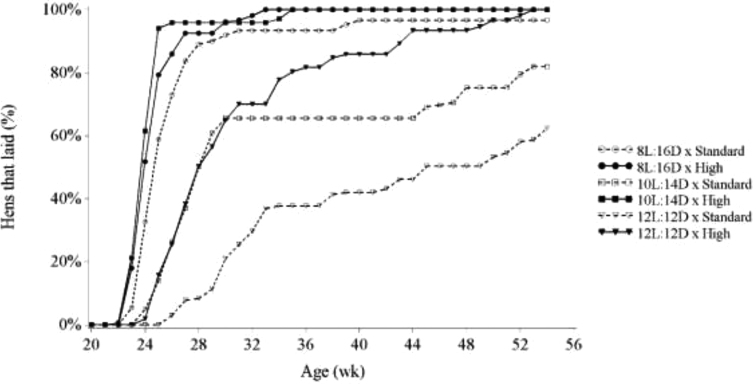
In contrast with Lewis (2006), we found that the effect of rearing photoschedule on sexual maturity and egg production was dependent on BW (Table 3; Figure 2). High BW × 8L:16D or 10L:14D hens did not differ in age at sexual maturity (173 vs. 172 d, respectively), but Standard BW hens showed a significant delay in sexual maturity when photoperiod increased from 8L:16D to 12L:12D (180 vs. 266 d, respectively), while the 10L:14D treatment was intermediate (212 d). In addition, Figure 2 shows that the difference in egg production within rearing photoschedule was greater for Standard BW hens compared with High BW hens. For the subset of hens that were laying, productivity did not differ between treatments (data not shown); thus, the difference in egg production originated from the rate (%) of hens reaching sexual maturity within each group (Figure 3). As a matter of fact, by the end of the experiment all High BW hens started laying, whereas within the Standard BW treatment 3.3, 18.1, and 37.6% of hens did not commence egg production under the 8L:16D, 10L:14D, and 12L:12D photoschedule, respectively (Table 3). In contrast with previous literature, the current study took a vastly different approach in the method of feeding used to control BW variation. First, the increased precision in which we were able to control BW and thus reduce BW CV with the PF system may have resulted in the ability to show the interaction between BW and rearing photoperiod. Second, the PF system provided an increased frequency of meals time-separated over the day and reduced meal size compared to conventional feeding methods. Feed allocation strategy also differed during the period where hens were expected to sexually mature, as there was no production-related feed increase. Feed allocations were provided to achieve the treatment-specific BW targets. Therefore, an individual hen would receive an additional feed allowance to support egg production only when her first egg was laid because in real-time, BW was reduced by the act of oviposition. We hypothesize that the combination of these factors might have altered the metabolism of the hens, potentially restricting hens to the point where metabolic triggers to sexually mature were absent or remaining suppressed, causing the interaction. This suggests that current breeder recommended BW targets may not allow for sufficient body reserves required for the onset of lay, at least in the PF scenario implemented in the current study.
Figure 3.
Percentage of hens that had laid their first egg. Hens were fed to achieve either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D rearing photoschedule.
Our results suggest that a stronger metabolic signal resulting from a higher positive energy balance in the High BW treatment countered negative signals caused by extended rearing photoperiods. A lower metabolic signal in the Standard BW treatment reduced reproductive axis responsiveness, and delayed sexual maturation. This hypothesis is consistent with the commercial practice of “challenge feeding.” The industry has observed for some time that increased feed intake allowance around the time egg production increases to 60 to 70% can stimulate egg production in the short term, presumably by bringing more hens into lay (Coon, 2002). The potential problem with challenge feeding is that it may reduce the persistency of lay for those hens already laying. Therefore, we suggest that it would be more advantageous to remove as many inhibitory reproductive signals as possible, instead of applying challenge feeding to synchronize sexual maturation.
As the integration center for the control of the reproductive axis is located within the hypothalamus, it has been suggested that the hypothalamus is a logical target for metabolic reproductive signals (Bédécarrats et al., 2016). Unpublished results from our laboratory indicate that hens with increased ME intake enter lay earlier with higher gonadotropin releasing hormone and lower gonadotropin inhibitory hormone expression in the hypothalamus compared to hens with a below average ME intake. Therefore, we hypothesize that whether direct or indirect metabolic cues can alter the balance between stimulatory and inhibitory output from the hypothalamus to the pituitary gland. However, further studies are required to identify the pathways and mechanisms involved.
Egg Weight
Egg weight increased with age for all treatments (P < 0.001), independent of BW treatment or rearing photoschedule (data not shown). Number of eggs less than 40 g and number of double yolked eggs did not differ between treatments (data not shown). There was a significant interaction between the effect of BW and rearing photoschedule on egg weight (P < 0.001). Egg weight was not different between the 8L:16D and 12L:12D photoschedules, but for the 10L:14D photoschedule, egg weight of Standard BW hens was 1.5 g lower compared with High BW hens (Table 3). No effects of rearing photoperiod on egg weight have been reported before. Previous studies showed that an increased 20 wk BW target did not affect egg weight (Fattori et al., 1991; Hocking et al., 2001, 2002; Gous and Cherry, 2004; Robinson et al., 2007; Ekmay et al., 2012; van Emous et al., 2013), where others showed that a 20% increased BW at 20 wk of age increased egg weight by about 1 g (Renema et al., 2001a, 2001b; Sun and Coon, 2005). We are currently unsure about the possible explanation of the described interaction.
Body Conformation
Proportional weight of body parts and number of LYF at 55 wk are reported in Table 4. Standard BW hens had a higher proportional breast weight and a lower proportional fatpad weight compared with High BW hens (27.5 vs. 25.8%; P = 0.006, and 1.5 vs. 2.4%; P < 0.001), respectively, which coincided with lower egg production. In addition, Standard BW hens had a lower proportional ovary weight and lower number of LYF compared to High BW hens. We hypothesize that lower egg production and delayed onset of lay in the Standard BW hens compared with High BW hens may have originated from an insufficient proportion or absolute amount of lean or fat mass. Although body part weights were not analyzed at photostimulation, we hypothesize that greater body mass accretion in High BW hens enhanced their reproductive readiness at the time of photostimulation, and these hens reached their minimum lean or fat mass thresholds earlier than Standard BW hens. Body composition, either (proportional) lean mass or fat mass, has been proposed as a factor partially responsible for age at sexual maturation and egg production in both laying hens and broiler breeders (Kwakkel et al., 1991, 1995; Lesuisse et al., 2017). Hens that did not commence egg production before week 55 had a 3.7% (of BW) greater proportion of breast and a 0.9% (of BW) smaller proportion of fatpad (Table 4). Thus, hens not in lay had 1.15 times more breast muscle and 0.63 of the abdominal fatpad of hens that had laid eggs. This suggests a deficiency of fat rather than a lean mass threshold. Therefore, a fat threshold mass required for the onset of lay may not have been achieved by almost one fifth of the Standard BW hens in our study.
Table 4.
Breast, fatpad, liver, heart, gastrointestinal tract (GIT), ovary, and oviduct weight as percentage of live BW, and number of large yellow follicles (LYF) of hens at 55 wk fed to achieve a High or Standard BW1 curve and reared to week 21 on an 8L:16D, 10L:14D, or 12L:12D photoschedule (RPS) and that either commenced egg production (laid) or did not commence egg production (not laid) before week 55.2
| BW | RPS | Breast (%) | SEM | Fatpad (%) | SEM | Liver (%) | SEM | Heart (%) | SEM | GIT (%) | SEM | Ovary (%) | SEM | Oviduct (%) | SEM | LYF | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | High | 25.8b | 0.64 | 2.4a | 0.26 | 1.7a | 0.09 | 0.36 | 0.017 | 4.8 | 0.15 | 1.6a | 0.22 | 0.9 | 0.12 | 4.4a | 0.47 | |
| Standard | 27.5a | 0.48 | 1.5b | 0.19 | 1.6b | 0.07 | 0.34 | 0.013 | 4.9 | 0.11 | 0.9b | 0.17 | 0.9 | 0.09 | 2.9b | 0.37 | ||
| RPS | 8L:16D | 26.1 | 0.68 | 2.1 | 0.27 | 1.9a | 0.09 | 0.38a | 0.018 | 5.0 | 0.15 | 1.3 | 0.24 | 0.9 | 0.13 | 3.8 | 0.49 | |
| 10L:14D | 27.2 | 0.62 | 1.7 | 0.25 | 1.7b | 0.08 | 0.35a,b | 0.016 | 4.8 | 0.14 | 1.1 | 0.22 | 0.9 | 0.11 | 3.9 | 0.46 | ||
| 12L:12D | 26.6 | 0.58 | 2.1 | 0.23 | 1.4c | 0.08 | 0.32b | 0.015 | 4.7 | 0.13 | 1.3 | 0.20 | 0.9 | 0.11 | 3.3 | 0.44 | ||
| BW × RPS | High | 8L:16D | 25.6 | 0.85 | 2.5 | 0.34 | 2.0 | 0.12 | 0.39 | 0.022 | 5.0 | 0.19 | 1.6 | 0.30 | 0.9 | 0.16 | 4.7 | 0.61 |
| 10L:14D | 26.6 | 0.85 | 2.2 | 0.34 | 1.7 | 0.12 | 0.37 | 0.022 | 4.7 | 0.19 | 1.4 | 0.30 | 0.9 | 0.16 | 4.8 | 0.61 | ||
| 12L:12D | 25.3 | 0.85 | 2.7 | 0.34 | 1.4 | 0.12 | 0.32 | 0.022 | 4.7 | 0.19 | 1.8 | 0.30 | 0.9 | 0.16 | 3.8 | 0.61 | ||
| Standard | 8L:16D | 26.6 | 0.82 | 1.6 | 0.33 | 1.8 | 0.11 | 0.38 | 0.021 | 5.1 | 0.19 | 1.1 | 0.29 | 0.8 | 0.15 | 3.0 | 0.57 | |
| 10L:14D | 27.9 | 0.73 | 1.3 | 0.29 | 1.6 | 0.10 | 0.34 | 0.019 | 5.0 | 0.17 | 0.7 | 0.26 | 0.9 | 0.14 | 3.1 | 0.55 | ||
| 12L:12D | 28.0 | 0.70 | 1.5 | 0.28 | 1.3 | 0.10 | 0.32 | 0.018 | 4.6 | 0.16 | 0.8 | 0.25 | 1.0 | 0.13 | 2.7 | 0.53 | ||
| Laid | 24.8b | 0.30 | 2.4a | 0.12 | 1.8a | 0.04 | 0.36 | 0.008 | 4.7 | 0.07 | 1.8a | 0.11 | 1.6a | 0.06 | 5.6a | 0.21 | ||
| Not laid | 28.5a | 0.93 | 1.5b | 0.37 | 1.5b | 0.13 | 0.34 | 0.024 | 5.0 | 0.21 | 0.7b | 0.32 | 0.2b | 0.17 | 1.8b | 0.72 | ||
| Source of variation | –––––––––––––––––––––––––––––––––––––––––––––––– P-value ––––––––––––––––––––––––––––––––––––––––––––––––––– | |||||||||||||||||
| BW | 0.006 | <0.001 | 0.044 | 0.379 | 0.587 | 0.001 | 0.912 | 0.001 | ||||||||||
| RPS | 0.253 | 0.347 | <0.001 | 0.002 | 0.064 | 0.556 | 0.930 | 0.323 | ||||||||||
| BW × RPS | 0.423 | 0.703 | 0.768 | 0.608 | 0.396 | 0.633 | 0.717 | 0.774 | ||||||||||
| Laid | <0.001 | 0.036 | 0.005 | 0.347 | 0.274 | 0.004 | <0.001 | <0.001 | ||||||||||
a–cLSMeans within a column and treatment group lacking a common superscript differ (P ≤ 0.05).
1Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
2 The effect of whether hens had commenced egg production or not before week 55 on body composition did not depend on rearing photoschedule.
Hens on the High BW treatment had similar egg production to the breeder performance objectives (Aviagen, 2016), which indicates that the Standard target BW curve was actually suboptimal, at least for precision-fed broiler breeders. High BW hens that weighed consistently less than the target BW were fed every time they entered the feeding station during the laying phase. Since these hens matured at a BW below the High target BW curve, their feed intake was unrestricted during lay. After meeting their maintenance ME requirements, their egg production potential would not have been limited by restricted ME intake, in contrast to what would have been the case for Standard BW hens.
The discrepancy between the performance of the hens on the Standard BW treatment and the breeder performance objectives (Aviagen, 2016) could have originated from differing body composition due to the feeding method. Zuidhof et al. (2015) showed that an increase in feeding frequency from skip-a-day to daily feeding increased breast muscle and reduced abdominal fat pad weights in breeder pullets. Compared to conventional feeding methods, feeding frequency in the current experiment was high, as the PF system fed individual hens small meals multiple times per day. Zuidhof et al. (2017) explained that high feeding frequency makes nutrients available from the gut throughout the day. This might reduce the metabolic incentive for hens to store energy in the form of fat and instead stimulate growth of lean body tissue. The energy stores in fat are required for egg production as production of yolk lipids and albumen protein requires energy. Therefore, insufficient fat stores in Standard BW hens could have delayed onset of lay. Consistent with this hypothesis, Lewis et al. (2003) compared hens from male and female breeder lines and showed that the leaner male line had a delayed sexual maturity compared to female line, independent of rearing photoschedule (223 vs. 207 d, respectively). In addition, in human medicine, there is a growing body of literature that describes that an early onset of sexual maturity coincides with an increased body fat percentage in women (Walvoord, 2010).
CONCLUSIONS
To our knowledge, this is the first time an interaction has been shown between the effects of BW and rearing photoperiod on reproductive performance in broiler breeders. These results suggest that greater BW or feed intake might override negative signals such as increased photoperiods against sexual maturation. In addition, increasing the target BW for breeder hens could increase egg production, particularly when managing BW with a PF system, and might counteract the negative effects of increased photoperiod during rearing in open housed facilities. We suggest further studies to investigate body fat thresholds in broiler breeders. In addition, we suggest further investigation into physiological and neuroendocrinological cues behind the effects of BW and rearing photoperiod.
ACKNOWLEDGMENTS
Financial support from Alberta Livestock and Meat Agency (Edmonton, Alberta), Ontario Ministry of Agriculture, Food and Rural Affairs (Guelph, Ontario) Canadian Poultry Research Council (Ottawa, Ontario), and Alberta Chicken Producers (Edmonton, Alberta) is gratefully acknowledged. Broiler breeder chicks were donated by Aviagen (Huntsville, Alabama). Lights were donated by Thies Electrical Distributing Co. (Cambridge, Ontario). The authors would like to acknowledge all volunteering students for their help during collection of the presented data. Special thanks to K. L. Lovely and C. A. Ouellette for their excellent technical support throughout the experiment. Thanks to the staff of the Poultry Research Centre (Edmonton, Alberta) for their technical support. Poultry Research Centre stakeholder contributions, which made this research possible, are gratefully acknowledged.
REFERENCES
- Aviagen. 2016. Ross 708 Parent Stock: Performance Objectives. Aviagen, Huntsville, AL. [Google Scholar]
- Bédécarrats G. Y., Baxter M., Sparling B.. 2016. An updated model to describe the neuroendocrine control of reproduction in chickens. Gen. Comp. Endocrinol. 227:58–63. [DOI] [PubMed] [Google Scholar]
- CCAC 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Coon C. N. 2002. Feeding broiler breeders. Pages 329–369 in Commercial Chicken Meat and Egg Production. Bell D. D., Weaver W. D. eds. Springer, Boston, MA. [Google Scholar]
- Ekmay R. D., Salas C., England J., Cerrate S., Coon C. N.. 2012. The effects of pullet body weight, dietary nonpyhtate phosphorus intake, and breeder feeding regimen on production performance, chick quality, and bone remodeling in broiler breeders. Poult. Sci. 91:948–964. [DOI] [PubMed] [Google Scholar]
- Fattori T. R., Wilson H. R., Harms R. H., Miles R. D.. 1991. Response of broiler breeder remales to feed restriction below recommended levels. 1. Growth and reproductive performance. Poult. Sci. 70:26–36. [DOI] [PubMed] [Google Scholar]
- Gous R. M., Cherry P.. 2004. Effects of body weight at, and lighting regimen and growth curve to, 20 weeks on laying performance in broiler breeders. Br. Poult. Sci. 45:445–452. [DOI] [PubMed] [Google Scholar]
- Hocking P. M. 2004. Roles of body weight and feed intake in ovarian follicular dynamics in broiler breeders at the onset of lay and after a forced molt. Poult. Sci. 83:2044–2050. [DOI] [PubMed] [Google Scholar]
- Hocking P. M., Bernard R., Robertson G. W.. 2002. Effects of low dietary protein and different allocations of food during rearing and restricted feeding after peak rate of lay on egg production, fertility and hatchability in female broiler breeders. Br. Poult. Sci. 43:94–103. [DOI] [PubMed] [Google Scholar]
- Hocking P. M., Maxwell M. H., Robertson G. W., Mitchell M. A.. 2001. Welfare assessment of modified rearing programmes for broiler breeders. Br. Poult. Sci. 42:424–432. [DOI] [PubMed] [Google Scholar]
- Kwakkel R. P., J. A. W., Van Esch, Ducro B. J., Koops W. J.. 1995. Onset of lay related to multiphasic growth and body composition in White Leghorn pullets provided ad libitum and restricted diets. Poult. Sci. 74:821–832. [DOI] [PubMed] [Google Scholar]
- Kwakkel R. P., de Koning F. L. S. M., Verstegen M. W. A., Hof G.. 1991. Effect of method and phase of nutrient restriction during rearing on productive performance of light hybrid pullets and hens. Br. Poult. Sci. 32:747–761. [DOI] [PubMed] [Google Scholar]
- Lesuisse J., Li C., Schallier S., Leblois J., Everaert N., Buyse J.. 2017. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult. Sci. 96:3949–3959. [DOI] [PubMed] [Google Scholar]
- Lewis P. D. 2006. A review of lighting for broiler breeders. Br. Poult. Sci. 47:393–404. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Backhouse D., Gous R. M.. 2004. Constant photoperiods and sexual maturity in broiler breeder pullets. Br. Poult. Sci. 45:557–560. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Backhouse D., Gous R. M.. 2005. Effect of constant photoperiods on the laying performance of broiler breeders allowed conventional or accelerated growth. J. Agric. Sci. 143:97–108. [Google Scholar]
- Lewis P. D., Ciacciariello M., Gous R. M.. 2003. Photorefractoriness in broiler breeders: sexual maturity and egg production evidence. Br. Poult. Sci. 44:634–642. [DOI] [PubMed] [Google Scholar]
- Payne C. G. 1975. Day-length during rearing and the subsequent egg production of meat-strain pullets. Br. Poult. Sci. 16:559–563. [Google Scholar]
- Renema R. A., Robinson F. E., Goerzen P. R.. 2001a. Effects of altering growth curve and age at photostimulation in female broiler breeders. 1. Reproductive development. Can. J. Anim. Sci. 81:467–476. [Google Scholar]
- Renema R. A., Robinson F. E., Goerzen P. R., Zuidhof M. J.. 2001b. Effects of altering growth curve and age at photostimulation in female broiler breeders. 2. Egg production parameters. Can. J. Anim. Sci. 81:477–486. [Google Scholar]
- Renema R. A., Rustad M. E., Robinson F. E.. 2007. Implications of changes to commercial broiler and broiler breeder body weight targets over the past 30 years. Worlds Poult. Sci. J. 63:457–472. [Google Scholar]
- Robinson F. E., Zuidhof M. J., Renema R. A.. 2007. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 1. Pullet growth and development. Poult. Sci. 86:2256–2266. [DOI] [PubMed] [Google Scholar]
- Romero L. F., Zuidhof M. J., Renema R. A., Robinson F. E., Naeima A.. 2009. Nonlinear mixed models to study metabolizable energy utilization in broiler breeder hens. Poult. Sci. 88:1310–1320. [DOI] [PubMed] [Google Scholar]
- Sun J., Coon C. N.. 2005. The effects of body weight, dietary fat, and feed withdrawal rate on the performance of broiler breeders. J. Appl. Poult. Res. 14:728–739. [Google Scholar]
- van Emous R. A., Kwakkel R. P., van Krimpen M. M., Hendriks W. H.. 2013. Effects of growth patterns and dietary crude protein levels during rearing on body composition and performance in broiler breeder females during the rearing and laying period. Poult. Sci. 92:2091–2100. [DOI] [PubMed] [Google Scholar]
- Walvoord E. C. 2010. The timing of puberty: Is it changing? Does it matter? J. Adolesc. Health 47:433–439. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Fedorak M. V., Kirchen C. C., Lou E. H. M., Ouellette C. A., Wenger I. I.. 2016. System and method for feeding animals. Precision ZX, Inc., assignee. United States Patent Application No. 15/283,125.
- Zuidhof M. J., Fedorak M. V., Ouellette C. A., Wenger I. I.. 2017. Precision feeding: Innovative management of broiler breeder feed intake and flock uniformity. Poult. Sci. 96:2254–2263. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Holm D. E., Renema R. A., Jalal M. A., Robinson F. E.. 2015. Effects of broiler breeder management on pullet body weight and carcass uniformity. Poult. Sci. 94:1389–1397. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Renema R. A., Robinson F. E.. 2007. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 3. Reproductive efficiency. Poult. Sci. 86:2278–2286. [DOI] [PubMed] [Google Scholar]