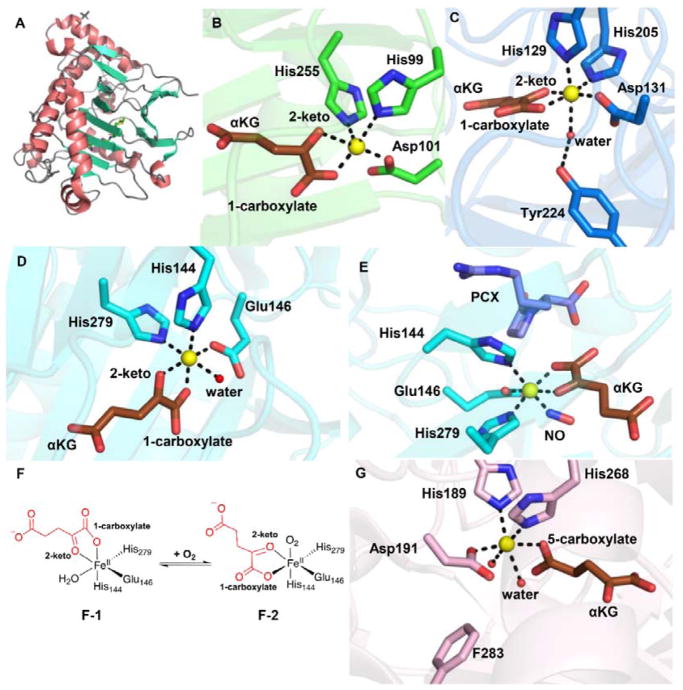
Fig. 2.
αKG-NHFe enzyme structural information. (A) The double-stranded helical fold (DSBH fold) first observed in IPNS structure.70 (B) A typical proximal αKG binding conformation represented by the TauD•Fe•αKG complex.77 (C) Distal-type αKG binding conformation represented by the FtmOx1•Fe•αKG complex.68 The proposed αKG conformational switch from the proximal (D) to the distal mode upon exposing the CAS•Fe•αKG•substrate complex to NO (E).78 (F) Schematic representation of αKG conformational switch between a proximal (F1)- and distal (F2)-type of conformation. (G) Another type of αKG binding conformation observed in the EFE•Fe•αKG binary complex where αKG binds to the Fe(II) centre monodentately using its C5 carboxylate.79 The iron centre is shown as yellow sphere, αKG is shown as brown sticks, water is shown as a red sphere, and NO is shown as sticks.