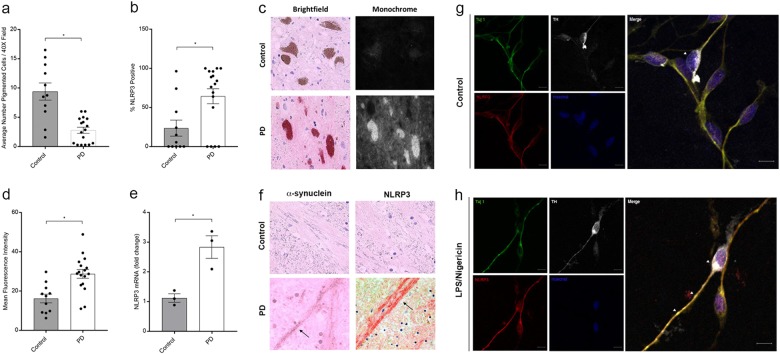
Fig. 1.
Elevated NLRP3 expression in human PD tissues. a Estimations of DA neuron density were determined by counting pigmented cell bodies across six 40× fields within the human substantia nigra in histologic sections obtained from control (n = 11) and PD patients (n = 17). The average number of pigmented neurons was significantly reduced in sections obtained from PD patients as compared with controls (*P < 0.001, two-tailed t test). b The percentage of NLRP3-positive cells determined in NLRP3 stained histologic sections. The average percentage of neuromelanin pigmented NLRP3-positive neurons was increased in sections obtained from PD patients (50 neurons counted per section, *P = 0.0413, two-tailed t test). c Immunohistologic sections obtained from the mesencephalon of controls (upper panels) and PD patients (lower panels) stained using anti-NLRP3 antibodies were viewed using both brightfield (left panels) and fluorescent excitation (595/Texas red filter and photographed in monochrome right panels). We used this method to assure specificity, as fluorescence specifically excites the fast red chromagen (lower right panel) and not endogenous neuromelanin (upper right panel). d Computer-assisted quantitation of mean per cell fluorescence was determined using a line profile approach (ImagePro). Mean NLRP3 immunofluorescence was elevated in cells of PD patients as compared with controls (12 representative neurons measured per section, control subjects (n = 11), PD patients (n = 17), *P < 0.001, two-tailed t test). e Total mRNA was isolated from representative cryopreserved mesencephalic tissues obtained from control (n = 3) and PD patients (n = 3), reverse transcribed, and analyzed using real-time PCR. NLRP3 mRNA was elevated in PD samples as compared with controls (*P < 0.01, two-tailed t test). Error bars represent s.e.m. f Immunohistologic sections obtained from the human mesencephalon of control subjects and PD patients were stained with anti-α-synuclein antibodies and anti-NLRP3 antibodies. No immunoreactivity was observed in DA fibers of the ventral substantia nigra in control samples. Lewy neurites were observed in sections obtained from PD patients immunostained using anti-α-synuclein antibodies (left panel, indicated with black arrow). NLRP3 immunoreactive neurites were only observed in PD patient samples (right panel, indicated with black arrow). g Confocal images of differentiated LUHMES cells treated with vehicle, fixed, immunostained with anti-Tuj1, anti-tyrosine hydroxylase (TH), and anti-NLRP3 antibodies, and counterstained with Hoechst dye. Scale bar represents 20 μM. h Differentiated LUHMES cells treated with LPS followed by Nigericin fixed and stained as described in g. Arrows indicate TH-positive neurons expressing NLRP3. Fluorescence intensity of Tuj1 and NLRP3 was measured in the cell body and axons from untreated and treated cells. NLRP3 fluorescence, normalized to Tuj1, was significantly greater in both the axons and cell bodies of LUHMES cells treated with LPS and Nigericin. Axons: mean intensity treated of 0.8643 ± 0.02401 vs. untreated 0.6256 ± 0.05577 (P = 0.0010, t test, n = 10 per image). Cell bodies: mean intensity treated of 0.8297 ± 0.02522 vs. untreated 0.6542 ± 0.02936 (P = 0.0003, t test, n = 10 per image)