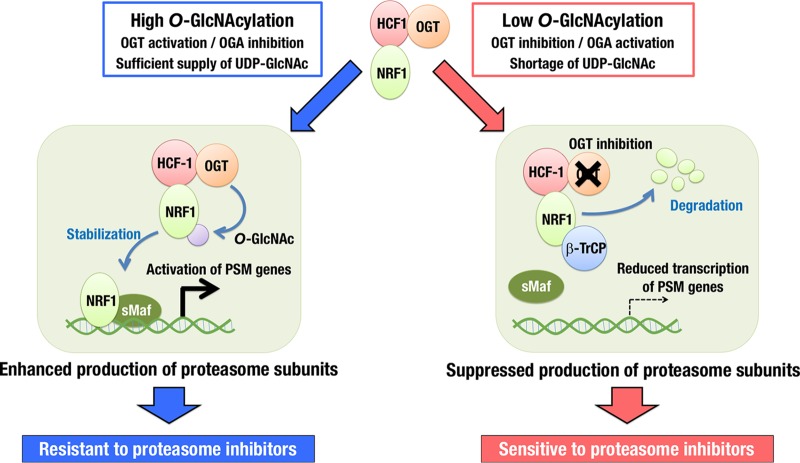
FIG 12.
Schematic illustration of O-GlcNAcylation switching on and off the activity of NRF1 for transcription of proteasome subunit (PSM) genes. With low O-GlcNAcylation activity, NRF1 preferentially interacts with β-TrCP and becomes ubiquitinated and degraded. With high O-GlcNAcylation activity, which is often observed in cancer cells, NRF1 is O-GlcNAcylated by its binding partner OGT/HCF-1 complex. O-GlcNAcylation of NRF1 disrupts the association between β-TrCP and NRF1, resulting in the enhancement of NRF1 accumulation, elevation of the PSM gene expression, and resistance to proteasome inhibitors. OGT inhibition effectively sensitizes cancer cells to proteasome inhibitors by suppressing the NRF1-mediated expression of PSM genes.