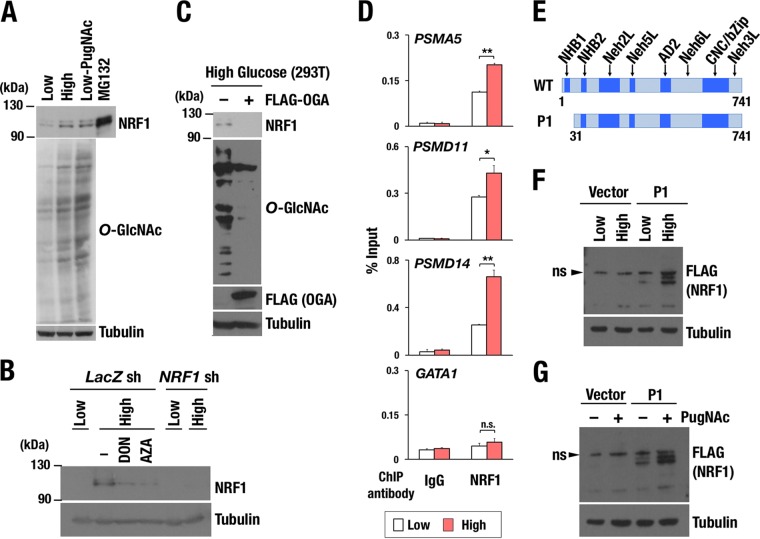
FIG 7.
Increased activity of cellular O-GlcNAcylation enhances accumulation of NRF1 protein. (A) Effect of O-GlcNAcylation on the protein level of endogenous NRF1. Whole-cell extracts were prepared from Hep3B cells that were cultured in medium containing 1.5 g/ml glucose (low), 4.5 g/ml glucose (high), or 1.5 g/ml glucose with 100 μM PugNAc (Low-PugNAc) for 24 h and subjected to immunoblot analysis with antibodies against NRF1, O-GlcNAc peptides, and tubulin. Hep3B cells that were treated with 10 μM MG132 were used as a positive control in NRF1 detection. (B) Effect of HBP inhibitors on the protein levels of endogenous NRF1. Whole-cell extracts were prepared from 293T cells expressing lacZ shRNA or NRF1 shRNA. Cells were cultured in low- or high-glucose medium with or without HBP inhibitors, 100 μM DON, or 100 μM AZA for 24 h before harvest. The protein extracts were subjected to immunoblot analysis with antibodies against NRF1 and tubulin. (C) Effect of OGA expression on the protein levels of endogenous NRF1. 293T cells were transfected with an empty or a FLAG-OGA expression vector. At 24 h after transfection, the cells were cultured in high-glucose medium for another 24 h and then harvested. Whole-cell extracts were prepared and subjected to immunoblot analysis with antibodies against NRF1, O-GlcNAc peptides, the FLAG tag, and tubulin. (D) Quantitative ChIP assay at the PSMA5, PSMD11, PSMD14, and GATA-1 gene loci in HeLa cells. Chromatin localization of NRF1 was examined in each sample that was treated with low or high glucose for 24 h. The values show the enrichment of immunoprecipitated DNA relative to input DNA. Averages and SD were calculated from triplicate samples. *, P < 0.05; **, P < 0.01. ns, not significant. (E) Constructs of 3×FLAG fusion proteins of NRF1 WT and P1 mutant (Δ30). (F and G) Accumulation of nuclear NRF1 (NRF1 P1) by enhancing cellular O-GlcNAcylation. 293F cells expressing 3×FLAG-NRF1 P1 were cultured in the medium containing low or high glucose (F) and treated with 100 μM PugNAc or left untreated (G). After 24 h, whole-cell extracts were prepared and subjected to immunoblot analysis with antibodies against the FLAG tag and tubulin.