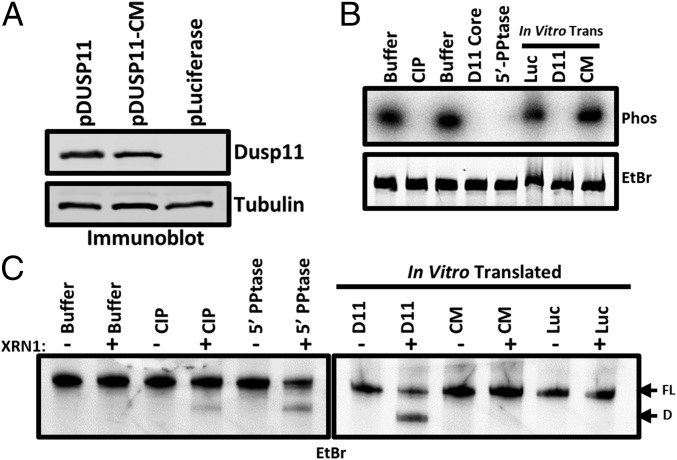
Fig. 4.
DUSP11 directly dephosphorylates HCV 5′ UTR RNA and sensitizes it to XRN-mediated degradation. (A) Confirmation of in vitro translation products by immunoblot. Membrane was incubated with the indicated antibodies. “pLuciferase” indicates reactions programmed to express luciferase as a negative control. (B) In vitro phosphatase assay. 5′ γ-32P–radiolabeled HCV 5′ UTR RNA was incubated with the indicated enzymes (calf intestinal phosphatase, purified DUSP11 core protein, and bacterial 5′ RNA polyphosphatase), or in vitro translated products from A (pDUSP11, pDUSP11-CM, and negative-control pLuciferase). Products were separated by urea/PAGE and stained with ethidium bromide. Products were then transferred to a nitrocellulose membrane and exposed to a phosphor storage screen (Phos). (C) In vitro XRN susceptibility assay. In vitro phosphatase reactions were performed as in B [calf intestinal phosphatase, bacterial 5′ RNA polyphosphatase, or in vitro translated products from A (pDUSP11, pDUSP11-CM, and negative-control pLuciferase)], but products were recovered and incubated ± recombinant XRN1. Products were separated by urea/PAGE and stained with EtBr. “FL” arrow points to the position of the full-length HCV 5′ UTR. “D” arrow points to the position of a faster-migrating degradation product.