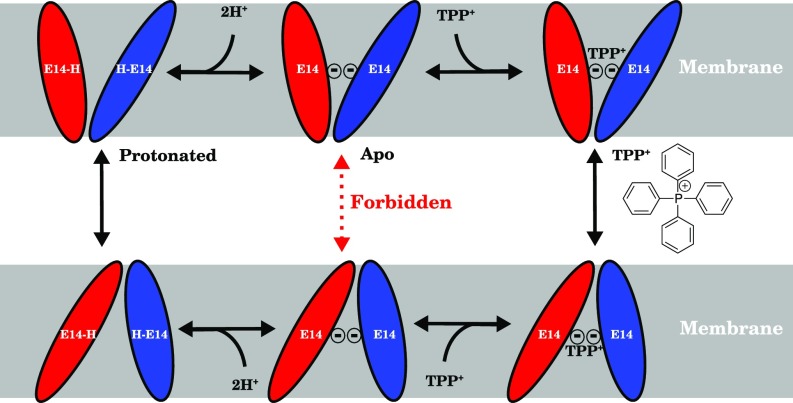
Fig. 1.
Schematic of the transport cycle of EmrE following the alternating access mechanism with a strict 2:1 : stoichiometry. EmrE monomers (red and blue ellipsoids) swap conformations (schematically indicated by the size and angle of the ellipse relative to the membrane) and thus alternate the substrate/proton accessibility to the inside or outside of the cell when loaded with protons (Left) or substrate (Right). When the EmrE dimer is in its apo (Center) form, the transition should be forbidden (red dashed arrow) to preserve the coupling of proton and substrate transport and prevent proton leakage across the membrane. This apo state is in contrast to both the fully protonated and substrate-bound states (Left and Right columns, respectively), whose transition should be allowed (black solid arrows) for EmrE to fulfill its physiological function as a proton-driven polyaromatic cation transporter.