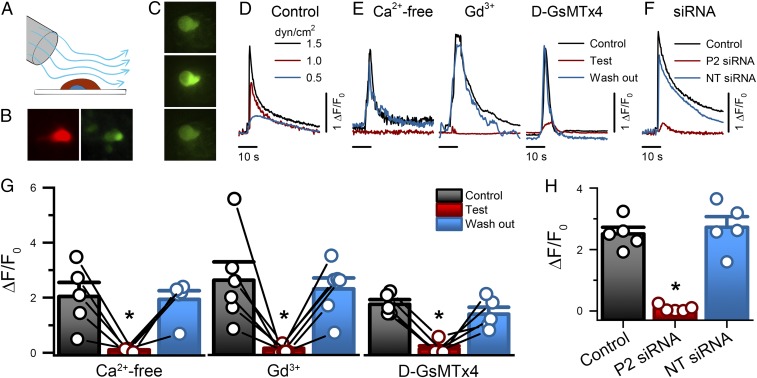
Fig. 4.
NeuroD1+ cell stimulation by shear force produces Piezo2-dependent transient intracellular Ca2+ increase. (A) The experimental set-up used to mechanically stimulate primary EE cells in culture using shear flow by rapid-perfusion system. (B) EE cells are identifiable by tdTomato (red) and GCaMP5 (green). (Magnification: 40×.) (C) Epifluorescence images of an EE cell GCaMP5 at rest (Top), during shear stimulation (Middle), and following recovery (Bottom). (Magnification: 40×.) (D) Representative EE cell GCaMP5 fluorescence fluctuations (ΔF/F0) elicited by transient shear force (0.5, 1.0, and 1.5 dyn/cm2). (E) Representative traces of GCaMP5 fluorescence in response to shear force (1.5 dyn/cm2) in the absence (Control, black), presence (Test, red), and following recovery (wash-out, blue) of Ca2+-free extracellular solution, 30 μM Gd3+, and 10 μM D-GsMTx4. (F) Representative traces of GCaMP5 fluorescence elicited by shear force in Piezo2 siRNA (P2 siRNA), NT siRNA, and Control cells. (G) Individual (circles) and mean ± SEM (bars) NeuroD1+ cell GCaMP5 responses (ΔF/F0) for Ca2+ free extracellular solution (control 2.0 ± 0.5, Ca2+-free 0.1 ± 0.02, wash-out 1.9 ± 0.3, n = 5), 30 µM Gd3+ (control 2.6 ± 0.7, Gd3+ 0.09 ± 0.05, wash-out 2.3 ± 0.4, n = 6) and 10 µM D-GsMTx4 (control 1.7 ± 0.2, D-GsMTx4 0.1 ± 0.1, wash-out 1.4 ± 0.2, n = 5). *P < 0.05, Tukey test with multiple comparisons. (H) Individual (circles) and mean ± SEM (bars) NeuroD1+ cell GCaMP5 responses (ΔF/F0) for nontransfected Control (2.5 ± 0.2, n = 5), Piezo2 siRNA-transfected (P2 siRNA, 0.08 ± 0.05, n = 5) and NT siRNA-transfected (2.7 ± 0.3, n = 5). *P < 0.05 for Piezo2 siRNA compared with control and NT siRNA, ANOVA with Bonferroni correction.