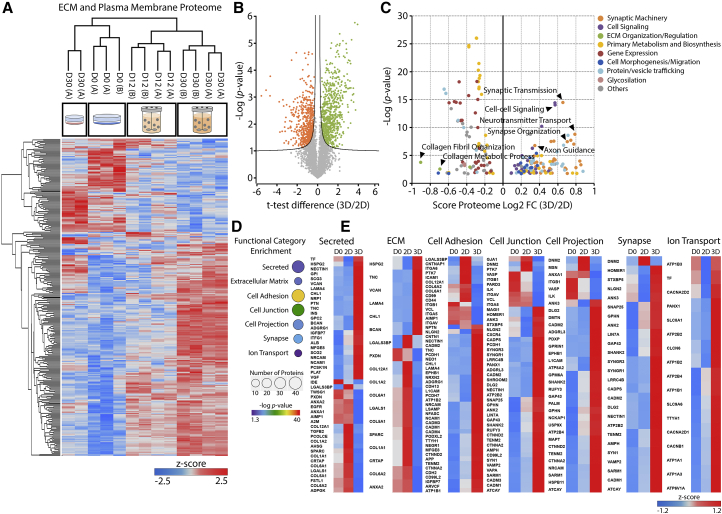
Figure 3.
Extracellular Space and Plasma Membrane Proteome Remodeling during hiPSC-NPC 3D Differentiation
(A) Heatmap of proteins significantly modulated at the extracellular or plasma membrane level, during 3D and 2D differentiation (total of 294 proteins). Hierarchical clustering performed for the biological replicates of each time point and cell line (in columns) and for proteins (in rows). Significantly modulated proteins identified by multi-sample ANOVA test with a permutation-based FDR cutoff of 0.05 applied on the logarithmized intensities. Z score values were color coded from blue (downregulation) to red (upregulation).
(B) Volcano plot of proteins identified after 2D or 3D differentiation of hiPSC-NPCs. Significantly enriched proteins after 2D (orange) or 3D (green) differentiation are color labeled. Significantly modulated proteins identified by permutation-based FDR t test applied on the logarithmized intensities, with a threshold value of 0.05 and S0 of 0.1.
(C) GO-BP terms significantly over-represented (Benjamini-Hochberg FDR < 0.02) in 2D (negative score) or 3D (positive score) differentiated cells. The y axis presents the corresponding p values (in negative log scale). Related GO-BP terms are presented with the same color, where some specific terms are highlighted by arrowheads.
(D) Selected functional categories significantly over-represented during 3D differentiation (Benjamini-Hochberg FDR < 0.05) for proteins annotated as extracellular space or plasma membrane components. p values and number of proteins are graphically represented by different colors and sphere sizes, respectively.
(E) Heatmaps of protein abundance profile at day 0 (D0), 2D at day 30, and 3D at day 30 of selected categories. Z score values were color coded from blue (downregulation) to red (upregulation). Data shown in all panels represent four (two independent experiments of two cell lines) or two pooled independent biological experiments, for neurospheroids or 2D cultures, respectively.