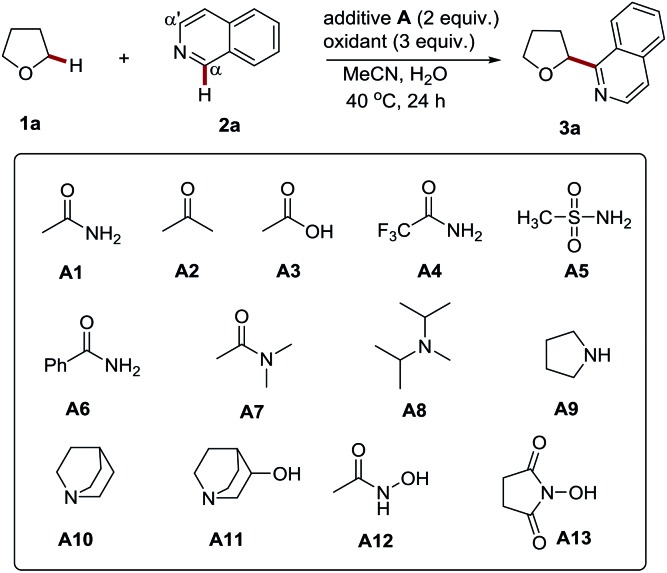
Table 1. Studies of the promotion effect of amides, their derivatives, amines and amine alcohols in the Cα-heteroarylation of tetrahydrofuran with isoquinoline and the optimization of the reaction conditions a .
| ||||
| Entry | Oxidant | Additive | 1a (equiv.) | Yield b |
| 1 | (NH4)2S2O8 | — | 20 | 14% |
| 2 | (NH4)2S2O8 | A1 | 20 | 77% |
| 3 | (NH4)2S2O8 | A2 | 20 | 15% |
| 4 | (NH4)2S2O8 | A3 | 20 | 24% |
| 5 | (NH4)2S2O8 | A4 | 20 | 50% |
| 6 | (NH4)2S2O8 | A5 | 20 | 53% |
| 7 | (NH4)2S2O8 | A6 | 20 | 55% |
| 8 | (NH4)2S2O8 | A7 | 20 | 70% |
| 9 | (NH4)2S2O8 | A8 | 20 | 7% |
| 10 | (NH4)2S2O8 | A9 | 20 | 15% |
| 11 | (NH4)2S2O8 | A10 | 20 | 80% |
| 12 | (NH4)2S2O8 | A11 | 20 | 82% |
| 13 | (NH4)2S2O8 | A12 | 20 | 5% |
| 14 | (NH4)2S2O8 | A13 | 20 | 88% |
| 15 | (NH4)2S2O8 | A13 | 20 | 42% c |
| 16 | (NH4)2S2O8 | A13 | 20 | 82% d |
| 17 | (NH4)2S2O8 | A13 | 20 | 75% e |
| 18 | (NH4)2S2O8 | A13 | 10 | 82% |
| 19 | (NH4)2S2O8 | A13 | 10 | 80% f |
| 20 | (NH4)2S2O8 | A13 | 5 | 60% f |
| 21 | (NH4)2S2O8 | A13 | 5 | 67% f , g |
aConditions employed 1a (2.5–10.0 mmol), 2a (0.5 mmol), oxidant (1.5 mmol), additive (1.0 mmol), 40 °C, 24 h, and a solvent mixture (1.5 mL, MeCN : H2O = 1 : 1), unless otherwise noted.
bThe yields were determined using 1H NMR with CH2Br2 as an internal standard.
cPerformed with (NH4)2S2O8 (1 equiv.).
dPerformed with A13 (1 equiv.).
ePerformed with A13 (0.5 equiv.).
fWater (1.5 mL) was used as the solvent.
gPerformed on a 1 g scale.
