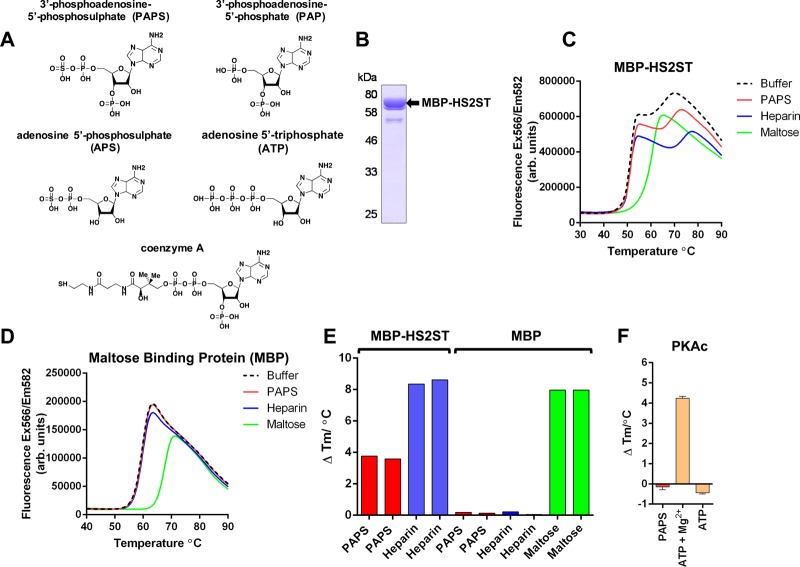
Figure 1. Analysis of purified recombinant MBP-HS2ST protein.
(A) Structures of PAPS and PAPS-related biochemicals. (B) Coomassie blue staining of recombinant MBP-HS2ST1 protein. Approximately 2 μg of purified enzyme was analysed after SDS–PAGE. (C) Thermal denaturation profiles of MBP-HS2ST (5 μM) and thermal shift in the presence of 0.5 mM PAPS (red), 10 μM heparin (blue) or 5 mM maltose (green). Buffer control is shown in black dashed lines. (D) Thermal denaturation profile of purified recombinant MBP. Experimental conditions as for (C). (E) Tm values measured for 5 μM MBP-HS2ST fusion protein (red, blue) or MBP (red, blue, green) in the presence of 0.5 mM PAPS, 10 μM heparin or 5 mM maltose. ΔTm values were obtained by DSF and calculated by subtracting control Tm values (buffer, no ligand) from the measured Tm. (F) ΔTm values relative to buffer addition for recombinant PKAc (5 μM) measured in the presence of 0.5 mM PAPS, 0.5 mM ATP or 0.5 mM ATP and 10 mM MgCl2. Similar results were seen in three independent experiments.