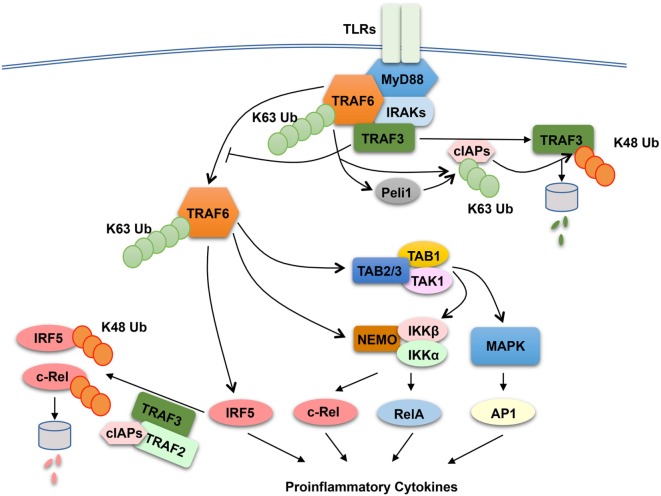
Figure 3.
Anti-inflammatory function of tumor necrosis factor receptor-associated factor (TRAF)2 and TRAF3 in toll-like receptor (TLR) signaling pathway. In responds to TLR stimulation, TRAF6 is recruited to the MyD88 signaling complex. Upon activation, the TRAF6 complex relocates from the plasma membrane to the cytoplasm, a signaling step required for activation of the downstream nuclear factor κB and mitogen-activated protein kinase (MAPK) pathways. TRAF3 negatively regulates TLR-stimulated proinflammatory cytokine induction via two potential mechanisms. The first is to target the MyD88–TRAF6 complex and prevents the relocation of TRAF6 to the cytoplasm, and the second is to function together TRAF2 and cIAPs to mediate ubiquitin-dependent degradation of two major proinflammatory transcription factors, c-Rel and interferon regulatory factor 5 (IRF5). TLR signaling temporarily overrides the negative signaling function of TRAF3 by inducing TRAF3 proteolysis. In this process, TRAF6 activates cIAPs (cIAP1 and cIAP2) through K63 ubiquitination, which in turn function as E3 ubiquitin ligases to mediate K48-linked ubiquitination and proteolysis of TRAF3. In microglia, Peli1 appears to cooperate with TRAF6 or function downstream of TRAF6 to mediate cIAP activation.