Fig. 2.
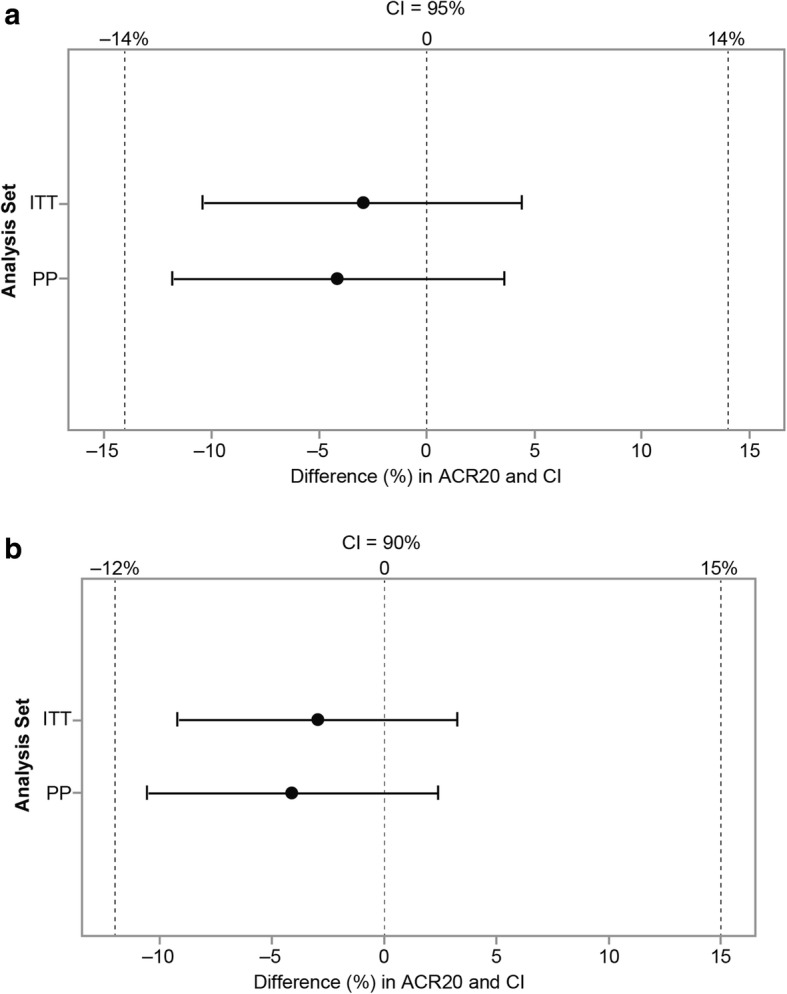
Primary efficacy endpoint of ACR20 at week 12 (with non-responder imputation). a Difference (95% CI) between PF-06410293 and adalimumab-EU using a symmetric equivalence margin. b Difference (90% CI) between PF-06410293 and adalimumab-EU using an asymmetric equivalence margin. Abbreviations: ACR20 American College of Rheumatology 20% improvement, Adalimumab-EU adalimumab sourced from the European Union, CI confidence interval, ITT intention-to-treat, PP per protocol
