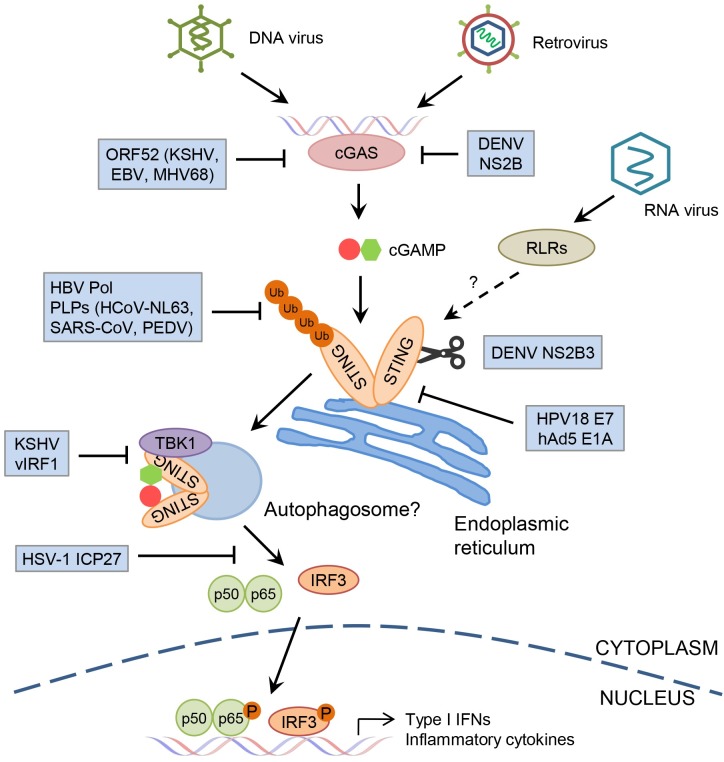
Fig 1. The cGAS–STING pathway and its counteraction by viruses.
Genomic DNA form DNA viruses or reverse transcription intermediates from retroviruses are recognized by cGAS, which catalyzes the production of cGAMP to bind and activate the ER-resident adaptor protein STING. STING then forms a complex with TBK1 and translocates from the ER to the perinuclear lysosomal compartments via an autophagy-like process. The STING–TBK1 complex subsequently activates transcription factors IRF3 and NF-κB to induce the production of type I IFNs and inflammatory cytokines to establish an antiviral state. Viruses have developed numerous strategies to antagonize the cGAS–STING pathway. Tegument protein ORF52 from gammaherpesviruses inhibits cGAS binding to viral DNA, while nonstructural protein NS2B of DENV promotes cGAS degradation. Similarly, DENV NS2B3 protease cleaves STING and leads to its degradation. HBV polymerase and papain-like proteases of human coronaviruses prevent or remove the K63-linked Ub of STING. KSHV vIRF1 blocks the TBK1-mediated phosphorylation of STING, while HSV-1 ICP27 prevents the phosphorylation of IRF3 by TBK1. HPV18 E7 protein and hAd5 E1A protein bind to STING and inhibit its activation. cGAMP, cyclic GMP-AMP; cGAS, cyclic GMP-AMP synthase; DENV, Dengue virus; ER, endoplasmic reticulum; HBV, Hepatitis B virus; hAd5, human adenovirus 5; HSV-1, herpes simplex virus 1; HPV18, human papillomavirus 18; ICP27, infected cell protein 27; IFN, interferon; IRF3, interferon regulatory factor 3; KSHV, Kaposi's sarcoma-associated herpesvirus; NF-κB, nuclear factor-κB; NS2B, nonstructural protein 2B; ORF52, open reading frame 52; P, phosphorylation; RLRs, RIG-I-like receptors; STING, stimulator of interferon genes; TBK1, TANK binding kinase 1; Ub, ubiquitination; vIRF1, viral interferon regulatory factor 1.