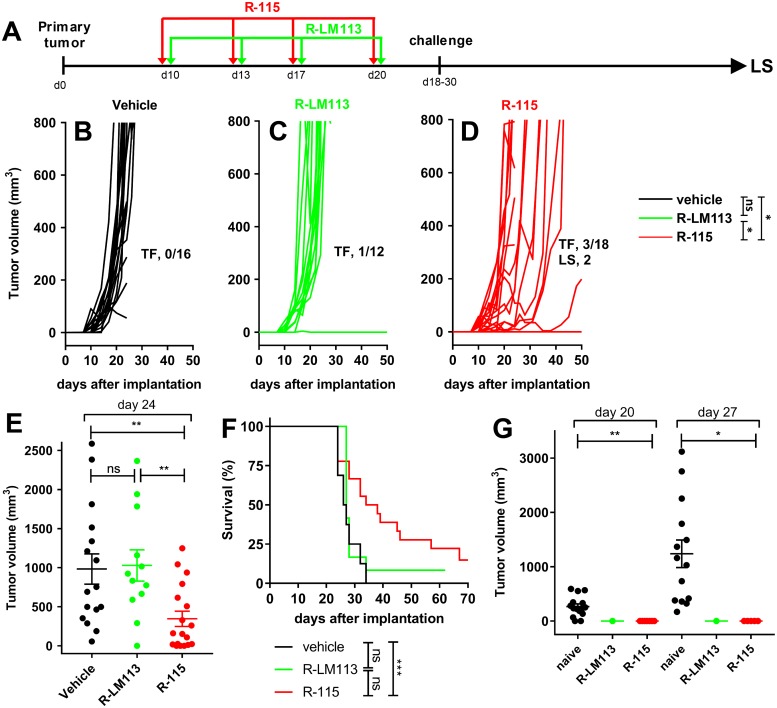
Fig 4. Efficacy of R-LM113 and R-115 administered 10 days after tumor implantation.
(A) Schedule of the treatments. HER2-transgenic/tolerant mice implanted with HER2-LLC1 tumor cells received four peri- intra-tumoral injections of R-LM113, R-115, or vehicle, at 3–4 days distance, starting at d 10 after tumor implantation. At d 18–30 they received a 1° contralateral challenge tumor. The mice which survived the primary tumor were all resistant to the 1° contralateral challenge tumor; a fraction of them was subsequently analyzed as long survivors (LS) (see, Fig 5). (B-D) Kinetics of tumor growth in mice treated with vehicle (B), R-LM113 (C), R-115 (D). Pooled results from 2 experiments. Statistical significance was calculated using the RM (repeated measures) two way ANOVA-test (until d 24). The figures in panels B-D denote the numbers of tumor free/treated (TF) mice, and the number of mice subsequently analyzed as LS. (E) Volumes of the primary tumors at d 24 after implantation. Statistical significance was calculated using the t-test. (F) Kaplan-Meier survival curves of the three groups of mice. Statistical significance was calculated using the Log-rank (Mantel-Cox) test. Of note, some tumor free mice were sacrificed during the course of the experiment in either arm and were censored. (G) Volumes of 1° contralateral untreated tumors in the R-LM113 and R-115 arms, and in naïve mice, at d 20 and 27 after its implantation. Statistical significance was calculated by means of the t-test. The number of mice in the naïve, R-LM113 and R-115 arms were 15, 1, 7, and 14, 1, 5 at d 20 and 27 after implantation of the contralateral tumor, respectively. The mice decreased in number because of deaths caused by the primary tumor. Two mice in the R-115 arm survived the primary tumor, received the 1° contralateral tumor and were included in the LS group (Fig 5).