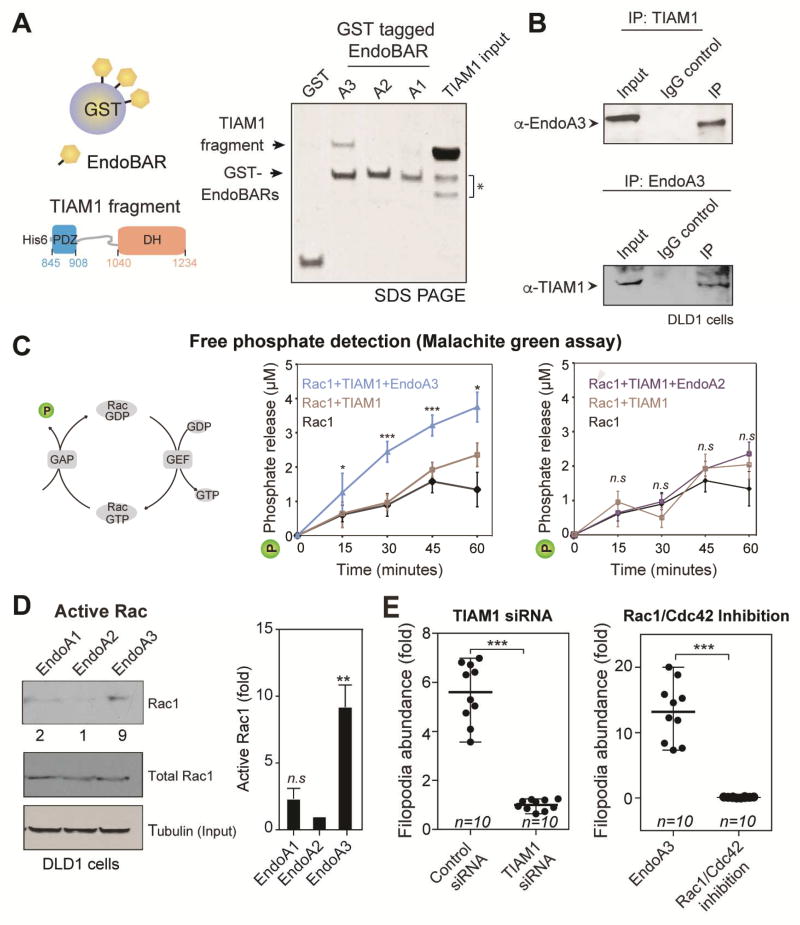
Figure 5. EndoA3 binds and activates TIAM1.
(A) EndoA3 shows robust binding with recombinant TIAM1. A scheme diagram of the GST pull-down assay is shown in the left panel. GST-tagged BAR domains of EndoA1, EndoA2, and EndoA3 (12 µg) were incubated with the recombinant TIAM1 fragment (20 µM, residues 841-1418) for 4 hours. Proteins bound to the GST beads were analyzed using SDS-PAGE and coomassie blue staining (right). “TIAM1 input” indicates 13% of the recombinant TIAM1 protein used in GST pull-down experiments. * indicates proteolytic fragments of the recombinant TIAM1. (B) Endogenous interactions between EndoA3 and TIAM1 in DLD1 cells. Co-immunoprecipitation experiments were performed using antibodies against EndoA3 (top) and TIAM1 (bottom). IgG was used as controls for immunoprecipitation experiments. Samples were analyzed using western blotting and enhanced chemiluminescence. (C) A scheme diagram showing that free phosphates (indicated as P) are released upon Rac1 GTP hydrolysis (left). The recombinant EndoA3 BAR domain (middle), but not the EndoA2 BAR domain (right), stimulates the TIAM1-dependent Rac1 GTPase activity in vitro. Free phosphates were detected using the malachite green absorbance. Unpaired Student’s t-tests were used for statistical analysis (n=5). *** p<0.001, ** p<0.01, and * p<0.05. (D) Expression of EndoA3 increases the levels of active Rac1 GTPase activity in DLD1 cells. Active Rac1 GTPases that bound to immobilized GST-tagged p21 activated kinase binding domain (PAK PBD) was detected using monoclonal anti-Rac1 antibody (Cytoskeleton Inc.) and enhanced chemiluminescence. Band intensities were quantified using densitometry (normalized to the EndoA2/tubulin ratio) (left). Increases of active RAC1 (fold) were quantified using data from three independent experiments (right). (E) Knockdown of TIAM1 (left) and the Rac1/Cdc42 inhibitor ML141 (right) blocked protrusion formation. Unpaired Student’s t-tests were used for 3 independent experiments (*** p<0.001).