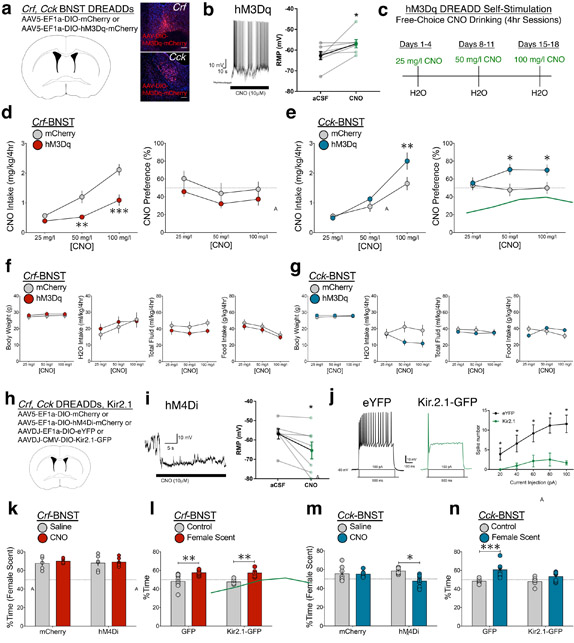
Figure 4. Crf and Cck BNST neurons: sufficiency and necessity for opposing motivated states.
(a) Crf- and Cck-Cre mice received bilateral BNST infusion of either control (mCherry) or excitatory DREADD (hM3Dq) virus, replicated independently with similar results in six mice per group. (b) Relative to aCSF, CNO application to BNST neurons elicited robust spiking activity and increased membrane potential (n=7 neurons from two mice; two-tailed paired t-test; t6 = 2.49, *p = 0.0472). (c) Timeline for CNO drinking hM3Dq self-stimulation studies. (d) CNO intake (left) and CNO preference (right) in Crf-BNST-hM3Dq and control mice (n=6 Crf-mCherry, n=12 Crf-hM3Dq-mCherry mice, two-way RM-ANOVA; intake: DREADD x CNO interaction: F2,32 = 5.32, p = 0.010, Bonferroni post-hoc comparisons **p < 0.005, ***p < 0.0005 vs. Crf-BNST-mCherry). (e) CNO intake (left) and CNO preference (right) in Cck-BNST-hM3Dq and control mice (n=15 Cck-mCherry, n=11 Cck-hM3Dq-mCherry mice, two-way RM-ANOVA; intake: DREADD x CNO interaction: F2,48 = 5.41, p = 0.008, Bonferroni post-hoc comparisons **p < 0.005 vs. Cck-BNST-mCherry). (f) Body weight, water intake, total fluid intake, and food intake for Crf BNST mice in CNO drinking studies (n=6 Crf-mCherry, n=12 Crf-hM3Dq-mCherry mice, no significant group x CNO interactions two-way RM-ANOVA DREADD x CNO interaction; p = 0.54, 0.52, 0.81, 0.96). (g) Body weight, water intake, total fluid intake, and food intake for Cck BNST mice in CNO drinking studies (n=15 Cck-mCherry, n=11 Cck-hM3Dq-mCherry mice, no significant group x CNO interactions two-way RM-ANOVA DREADD x CNO interaction; p = 0.62, 0.13, 0.69, 0.14). (h) For chemogenetic inhibition experiments, Crf- and Cck-Cre mice received bilateral BNST infusion of either control (mCherry) or inhibitory DREADD (hM4Di) virus. For physiological inhibition experiments, Crf- and Cck-Cre mice received bilateral BNST infusion of either control (eYFP) or inhibitory K+ channel (Kir2.1) virus. (i) Relative to aCSF, CNO application to hM4Di-expressing BNST neurons reduced spiking activity and significantly decreased resting membrane potential (n=7 neurons from two mice; two-tailed paired t-test; t6 = 2.59, *p = 0.0412). (j) Relative to eYFP-expressing control BNST neurons, Kir2.1-infected BNST neurons showed reduced spiking activity in response to increasing current injection (n=5 neurons from two mice per group; Current x Virus interaction: F4,32 = 3.1, *p = 0.029, Bonferroni post-hoc comparisons *p < 0.05). (k) Behavioral approach to female mouse odorant stimuli in male Crf-BNST mice following hM4Di DREADD inhibition (n=5 Crf-mCherry, n=6 Crf-hM4Di-mCherry, two-way RM-ANOVA; DREADD x CNO interaction: F1,9 = 0.12, p = 0.737). (l) Behavioral approach to female mouse odorant stimuli vs. control no-scent stimuli in male Crf-BNST mice following Kir2.1 manipulation (n=8 Crf-GFP, n=6 Crf-Kir2.1-GFP mice, two-way RM-ANOVA; Kir2.1 x stimuli interaction: F1,12 = 0.01, p = 0.912, Bonferroni post-hoc comparisons **p < 0.01 vs. no-scent control). (m) Behavioral approach to female mouse odorant stimuli in male Cck-BNST mice following hM4Di DREADD inhibition (n=7 per group, two-way RM-ANOVA; DREADD x CNO interaction: F1,12 = 4.98, p = 0.046, Bonferroni post-hoc comparisons *p < 0.05 vs. saline control. (n) Behavioral approach to female mouse odorant stimuli vs. control no-scent stimuli in male Cck-BNST mice following Kir2.1 manipulation (n=8 per group, two-way RM-ANOVA; Kir2.1 x stimuli interaction: F1,14 = 3.73, p = 0.074, Bonferroni post-hoc comparisons ***p < 0.0005 vs. no-scent control). In panels b, d–g, and i–n, centre and error bars are mean ± S.E.M. Scalebars: all 100 μm.