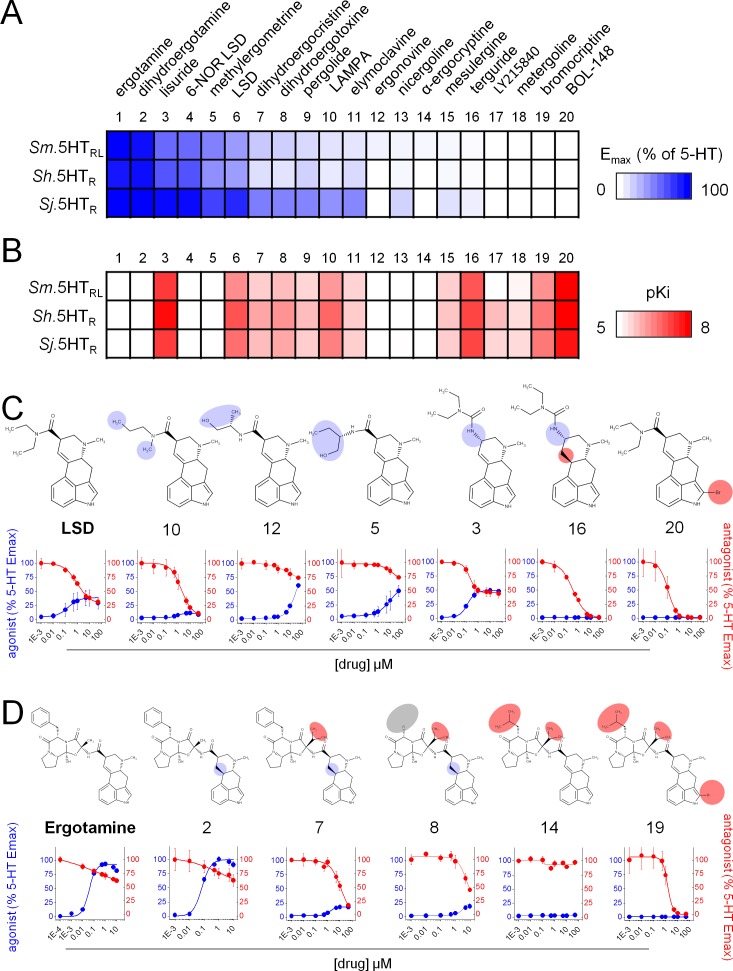
Figure 3. Structure activity relationship of ergot alkaloid compounds.
Ergot alkaloids display a spectrum of activity against Schistosoma 5-HT receptors ranging from (A) agonists (expressed as maximal cAMP response relative to 5-HT) to (B) antagonists (estimated receptor affinity expressed as pKi). (C) Structure activity relationship within a lysergic acid amide series. Derivatives of the partial agonist LSD displayed modified activity following modification of the diethylamide moieties (Danso-Appiah and De Vlas, 2002; Fallon and Doenhoff, 1994; Cheever, 1969; Wilson, 2009) or the ergoline ring system (Geary et al., 1992; Chan et al., 2016c). (D) Structure activity relationship within a ergopeptine chemical series. Derivatives of the full agonist ergotamine with altered activity following modification of the tripeptide structure (Barlow and Meleney, 1949; Andrews et al., 1983; Ismail et al., 1996) or the ergoline ring system (Chan et al., 2016b). Compound numbering reflects rank order of efficacy across the schistosome 5-HTRs: 1. Ergotamine; 2. Dihydroergotamine; 3. Lisuride; 4. 6-NOR LSD; 5. Methylergometrine; 6. LSD; 7. Dihydroergocristine; 8. Dihydroergotoxine; 9. Pergolide; 10. LAMPA; 11. Elmoclavine; 12. Ergonovine; 13. Nicergoline; 14. α-ergocryptine; 15. Mesulergine; 16. Terguride; 17. LY215840; 18. Metergoline; 19. Bromocriptine; 20. BOL-148. Data represent mean ± standard error of at least three biological replicates.