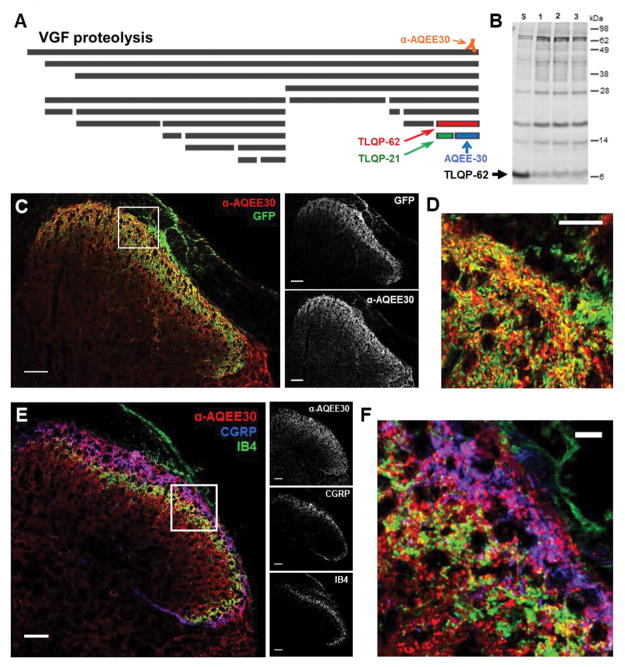
Figure 1. Spinal VGF is derived from multiple sources and the C-terminal peptide TLQP-62 is present in spinal cord.
A) The neurosecretory protein VGF undergoes proteolytic processing to yield multiple bioactive peptides. The C-terminal VGF peptide antibody, anti-AQEE30, was generated against a peptide containing the final 30 amino acids in the VGF sequence (AQEE30), and binds all VGF fragments containing the C-terminus. B) Western blot analysis of spinal cord lysates with anti-AQEE30 demonstrates the presence of TLQP-62 in the spinal cord (Lane S, spinal cord lysate spiked with 0.125 pmol TLQP-62 peptide standard; Lanes 1–3, spinal cord lysates from 3 naïve mice). C) Localization of anti-AQEE30 immunoreactivity to central primary afferent processes by GFP co-immunolabeling in Pirt-GCamp3 mice. Scale bars = 50 μm. D) Zoomed in image of the area outlined by the white box in C illustrates extensive colocalization of anti-AQEE30 and GFP immunolabeling. Scale bar = 10 μm. E) Localization of anti-AQEE30 immunoreactivity to central primary afferent processes by co-labeling for CGRP and IB4. Scale bars = 50 μm. F) Zoomed in image of the area outlined by the white box in E. Scale bar = 10 μm.