Figure 5.
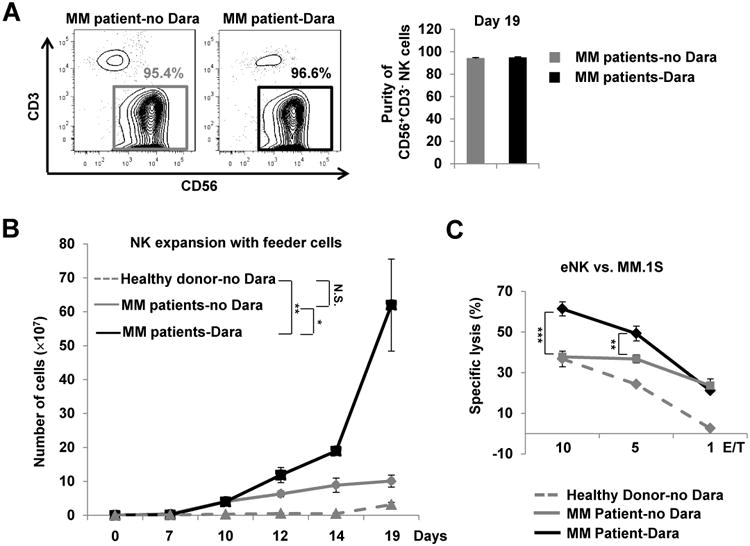
Expansion of primary NK cells from MM patients' PBMCs. A, Purity of NK cells expanded (eNK) from non-Dara-treated or Dara-treated MM patients assessed by flow cytometry at day 19 and presented as the percentage of CD56+CD3− lymphocytes among total lymphocytes (n=3). B, Expansion of NK cells derived in vitro from the PBMCs of healthy donors and MM patients treated with or without Dara (Dara and no Dara, respectively), as assessed on days 7, 10, 12, 14 and 19 (n=3). C, A 4-h standard 51Cr-release assay was performed using NK effector cells expanded from the PBMCs of healthy donors or MM patients treated with or without Dara (n=3). MM.1S served as targets. Dara, daratumumab; Error bars, S.D; N.S., not significant; *, P< 0.05;**,P< 0.01; ***, P< 0.001.
