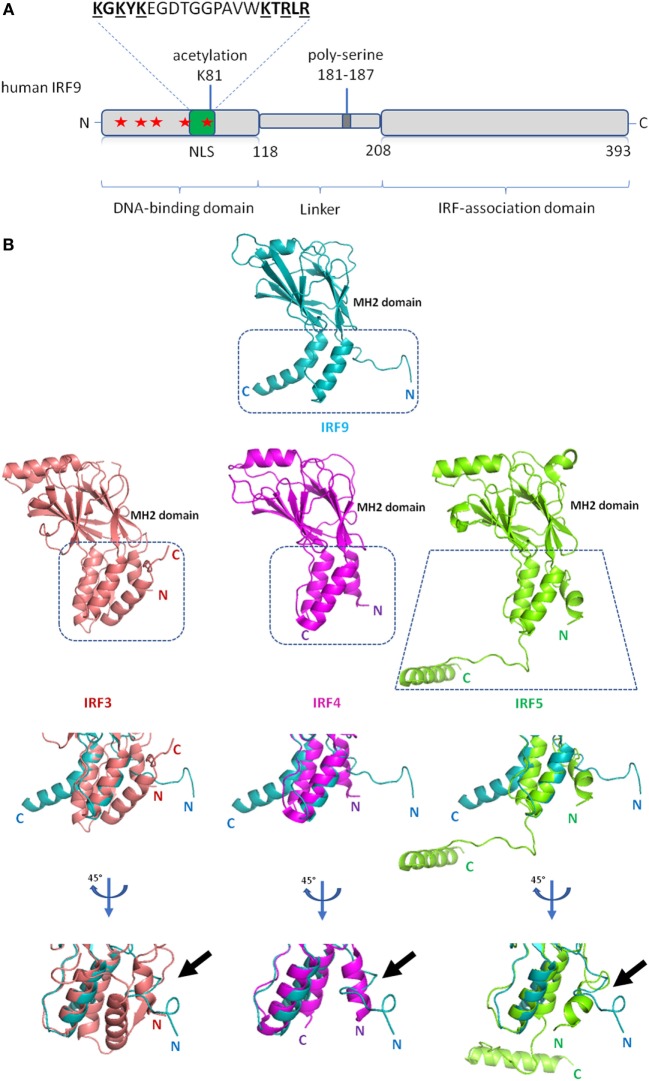
Figure 2.
Schematic diagram of interferon regulatory factor 9 (IRF9) and structure of IRF9 IRF-associated domain (IAD). (A) Domain organization of the full length human IRF9 shown in a schematic representation. The conserved tryptophan pentad (labeled red stars) of IRF9 are located at amino acid positions 15, 30, 42, 62, and 80 within the DNA-binding domain. Green box indicates the position (a.a. 66–85) of nuclear localization signal (NLS) of IRF9. The largely basic bipartite NLS is characterized as KGKYK separated by a spacer sequence of 10 amino acids followed by KTRLR (basic amino acids are shown underlined). (B) Crystal structures of the IAD of IRF9 [Protein Data Bank (PDB) ID code 5OEM], IRF3 (PDB ID code 3A77), IRF4 (PDB ID code 5BVI), IRF5 (PDB ID code 3DSH) show similarity in tertiary structure between all four proteins. The Mad-homology 2 fold (β-sheets, center core) is visibly conserved in the IAD of all four IRFs. Close-up structural superposition between IRF9 against IRF3, IRF4, and IRF5 disclose the absence of N-terminal autoinhibitory helical structure (α1 helix) in the IAD of IRF9 (see black arrow). Therefore, IRF9 could be constitutively active.