Abstract
A 19-year-old man was referred due to sudden onset of right foot pain and chest discomfort. Contrast-enhanced computed tomography revealed massive thrombi in the right pulmonary artery and femoral vein. The patient's father had experienced multiple recurrences of venous thromboembolism (VTE) and was diagnosed with inherited antithrombin deficiency by a genetic examination. The patient was administered the oral factor Xa inhibitor rivaroxaban (30 mg). After seven days, the thrombus disappeared. Rivaroxaban (15 mg) was continued for 6 months with no recurrence, indicating the efficacy of this factor Xa inhibitor for the treatment and prevention of VTE in patients with antithrombin deficiency.
Keywords: antithrombin deficiency, venous thromboembolism, factor Xa inhibitor
Introduction
Inherited antithrombin (AT) deficiency is an autosomal dominant disorder with an estimated prevalence of 0.02-0.2% (1). Patients with AT deficiency are at a substantially increased risk of venous thromboembolism (VTE), including deep venous thromboembolism (DVT) and pulmonary embolism (PE). The recommended initial treatment for VTE is the continuous administration of heparin or fondaparinux. However, in patients with AT deficiency, there are potential risks of heparin resistance and thrombus progression because of the low activity of AT (2). Recently, oral factor Xa (FXa) inhibitors have been proven effective for treating VTE (3); however, the experience of their use in patents with AT deficiency is limited.
We herein report a case of PE and DVT in a patient with inherited AT deficiency, in which the FXa inhibitor rivaroxaban was markedly effective.
Case Report
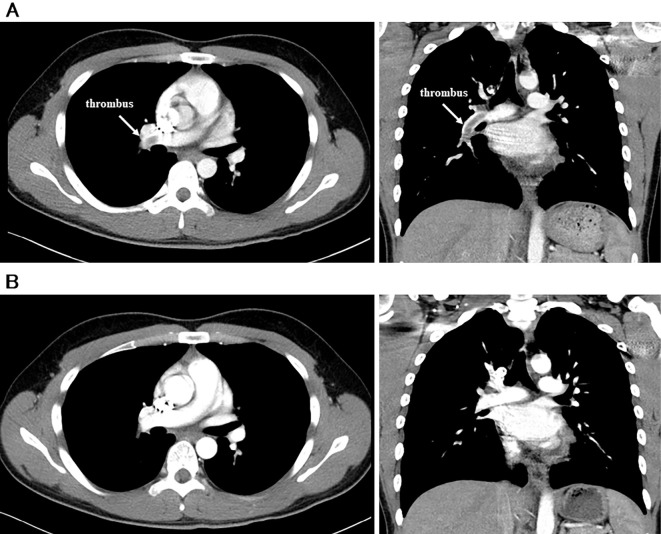
A 19-year-old man was referred to our center with the sudden onset of right foot pain and chest discomfort. His father and grandmother had a history of DVT and PE, and his father was diagnosed with inherited AT deficiency by a genetic examination. His vital signs on arrival were as follows: heart rate of 110 bpm, blood pressure of 92/64 mmHg, respiratory rate of 24 breaths per minute and oxygen saturation of 95% with 5 L per minute of supplemental oxygen. A physical examination showed prominent IIp sound. Contrast-enhanced computed tomography revealed massive thrombi in the right pulmonary artery and the right femoral vein (Fig. 1A). The patient's serum D-dimer level was elevated (42 μg/mL), and the AT activity and AT antigen level were markedly low (38%, 9.2 mg/dL). Protein C and protein S plasma levels were within the normal range, or no lupus anticoagulant and anticardiolipin antibodies were detected.
Figure 1.
Contrast-enhanced computed tomography scans. (A) Massive thrombi were detected in the right pulmonary artery on admission. (B) Seven days after the use of rivaroxaban 30 mg (15 mg twice a day), the thrombi had disappeared. (left: horizontal view, right: coronal sectional view).
Based on the familial history and findings from several examinations, this patient was diagnosed with PE and DVT with inherited AT deficiency. The treatment of PE in the acute phase has been reported to be as follows: hemodynamic and respiratory support, anticoagulation therapy, percutaneous catheter-directed treatment, thrombolytic treatment and surgical embolectomy (4). Surgical embolectomy and percutaneous catheter-directed treatment were not considered as the first-line therapy in this case because the patient was considered not to be in shock. Thrombolytic treatment using a recombinant tissue plasminogen activator was refused by the patient's parents due to the risk of bleeding complications. Because of the low AT activity, heparin or fondaparinux and transition to a vitamin K antagonist therapy might have taken time to achieve a therapeutic anticoagulant effect. After obtaining written informed consent, we started rivaroxaban 30 mg (15 mg twice a day) for 3 weeks and then reduced the dose to 15 mg per day. Two days after admission, he no longer had foot pain or chest discomfort, and his vital signs dramatically improved as follows: heart rate 66 bpm, blood pressure of 124/72 mmHg, oxygen saturation of 97% without supplemental oxygen. Seven days after admission, the thrombus had disappeared (Fig. 1B).
Because coagulation tests can be unreliable in the acute phase of VTE, several coagulation tests were performed after discharge. The AT functional activity and antigen level remained low (47%, 10.1 mg/dL), and a genetic examination revealed inherited type I AT deficiency. With 15 mg rivaroxaban daily after 30 mg for 3 weeks, he experienced no recurrence of PTE or DVT during the 10-month follow-up.
Discussion
This case indicated the efficacy of an FXa inhibitor for VTE in a patient with AT deficiency. Patients with AT deficiency are at significantly increased risk for VTE and the onset of thrombotic events occurs between 10 and 35 years of age in 67% of patients with hereditary AT deficiency (5). Approximately 50-90% of patients with AT deficiency develop VTE during their life-time (6). The efficacy and safety of rivaroxaban for VTE was well-demonstrated in the EINSTEIN study (7); however, its efficacy in patients with inherited AT deficiency has not been well established. To our knowledge, this case is the first showing the efficacy of FXa inhibitor for treatment in the acute phase and preventing recurrences of VTE in a patient with AT deficiency.
This case raised two clinically important issues about VTE therapy in patients with AT deficiency. First, FXa inhibitors can exert an anticoagulant effect not influenced by the low AT activity (8). AT is a potent inactivator of thrombin and factor Xa and a major inhibitor of blood coagulation (Fig. 2). Rivaoxaban may act directly without the participation of AT and thereby provide a valid anticoagulant effect, particularly for AT-deficient patients. Second, FXa inhibitors can result in a faster onset of anticoagulation than heparin and transition to a vitamin K antagonist (9). Heparin works as an anticoagulant through the potentiation of endogenous AT. Patients with AT deficiency may therefore experience resistance to therapy with heparin and may require higher heparin doses. A vitamin K antagonist may also need time to produce an anticoagulant effect. The initial use of an FXa inhibitor can quickly produce an anticoagulant effect without AT or the risk of heparin resistance or thrombus progression.
Figure 2.
Natural anticoagulants cascade and various anticoagulant agents. Antithrombin is an inactivator of thrombin and factor Xa and a major inhibitor of blood coagulation. Heparin and its derivatives act by increasing the activity of antithrombin. Rivaroxaban directly binds to the active site of Xa, blocking the activity. AT: antithrombin, II: prothromin, IIa: thrombin, UFH: unfractionated heparin, LMWH: low-molecular-weight heparin
The efficacy of AT replacement therapy for patients with inherited AT deficiency has been described in several case reports (10, 11). However, no randomized clinical trials have been reported assessing the efficacy for AT replacement therapy and no consensus statements exist as to when to use AT concentrates and when not to use them (6). In addition, AT concentrates are expensive.
The possible utility of other direct oral anticoagulants, especially other FXa inhibitors, for the treatment and prevention of VTE in patients with AT deficiency is worth discussing. An animal experimental study suggested that edoxaban, another FXa inhibitor, might be effective in patients with low plasma AT concentrations (12). Kawano et al. reported a case report on the efficacy of edoxaban for VTE in a cancer patient with AT deficiency (13). Theoretically, other FXa inhibitors should also be effective in VTE patients with AT deficiency. Inherited AT deficiency is dived into type I deficiency, in which both the functional activity and antigenic levels of AT are reduced, and type II deficiency, in which normal antigen levels are found with low AT activity. Type II deficiency is further divided into three types; IIa, IIb, and IIc, according to their site of defect as determined by a genetic analysis (14). The present patient was revealed to type I AT deficiency based on AT activity and antigen assay and a genetic examination. It has been reported that type I hereditary AT deficiency is associated with a greater risk of VTE than type II AT deficiency and other thrombophilias (15).
However, there are no guidelines nor any consensus about how long oral anticoagulants should be continued in order to prevent the recurrence of VTE in patients with AT deficiency. The risk of recurrent VTE in patients with AT deficiency not treated with long-term anticoagulation is reportedly high (16). The present patient experienced no recurrence with 15 mg rivaroxaban for 10 months. Therefore, FXa inhibitors may be effective preventing VTE recurrence in patients with AT deficiency. Our patient may require oral anticoagulant throughout his life because he has symptomatic type I AT deficiency, which is associated with a higher risk of VTE recurrences than other thrombophilias.
Rivaroxaban proved to be effective for the treatment and prevention of the recurrence of VTE in patients with AT deficiency. Further studies involving a greater number of cases are needed to clarify the efficacy of FXa inhibitors for VTE in patients with AT deficiency.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Tait RC, Walker ID, Perry DJ, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol 87: 106-112, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Spiess BD. Treating heparin resistance with antithrombin or fresh frozen plasma. Ann Throrac Surg 85: 2153-2160, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism; a systematic review and meta-analysis. J Thromb Haemost 12: 320-328, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinides SV, Torbicki A, Agnelli G, et al. Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J 35: 3033-3069, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Thaler E, Lechner K. Antithrombin III deficiency and thromboembolism. Clin Haematol 10: 369-390, 1981. [PubMed] [Google Scholar]
- 6.Rodgers GM. Role of antithrombin concentrate in treatment of hereditary antithrombin deficiency. An update. Thromb Haemost 101: 806-812, 2009. [PubMed] [Google Scholar]
- 7.Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363: 2499-2510, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia 14: 1229-1239, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Skelley JW, White CW, Thomason AR. The use of direct oral anticoagulants in inherited thrombophilia. J Thromb Thrombolysis 43: 24-30, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Konkle BA, Bauer KA, Weinstein R, Greist A, Holmes HE, Bonfiglio J. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Transfusion 43: 390-394, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Menache D, O'Malley JP, Schorr JB, et al. Evaluation of the safety, recovery, half-life, and clinical efficacy of antithrombin III (human) in patients with hereditary antithrombin III deficiency. Blood 91: 4561-4571, 1998. [PubMed] [Google Scholar]
- 12.Fukuda T, Kamisato C, Honda Y, et al. Impact of antithrombin deficiency on efficacy of edoxaban and antithrombin-dependent anticoagulants, fondaparinux, enoxaparin, and heparin. Thromb Res 131: 540-546, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Kawano H, Maemura K. Edoxaban was effective for the treatment of deep vein thrombosis and pulmonary thromboembolism in a cancer patient with antithrombin III deficiency. Intern Med 55: 3285-3289, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finazzi G, Caccia R, Barbui T. Different prevalence of thromboembolism in the subtypes of congenital antithrombin III deficiency: review of 404 cases. Thromb Haemost 18: 1094, 1987. [PubMed] [Google Scholar]
- 15.Martinelli I, Mannucci PM, De Stefano V, et al. Different risk of thrombosis in four coagulation defects associated with inherited thrombophilia: a study of 150 families. Blood 92: 2353-2358, 1998. [PubMed] [Google Scholar]
- 16.van den Belt AG, Sanson BJ, Simioni P, et al. Recurrence of venous thromboembolism in patients with familial thrombophilia. Arch Intern Med 157: 2227-2232, 1997. [DOI] [PubMed] [Google Scholar]