Abstract
Bufalin has been demonstrated to possess a wide range of pharmacological effects. Among these is its antitumour effect, which has been confirmed in multiple organs or tissues and provoked many concerns. However, its cytostatic effect and underlying mechanism in bladder cancer has not thoroughly been elucidated. This study aimed to investigate the hypothesis that Bufalin induces cell apoptosis and inhibits cell growth in bladder cancer through the inactivation of Na+/K+-ATPase (NKA). In the current study, it was demonstrated that Bufalin remarkably inhibited cell viability and induced cell apoptosis in bladder cancer cell line T24. Subsequently, we found that the expression of NKA was significantly supressed in Bufalin-treated cells and the NKA-α3 isoform was most sensitive to Bufalin among all α subunits of NKA. By transfection with NKA-α3 overexpressing plasmids, the expression of the NKA-α3 subunit was upregulated and NKA-α3 overexpression was found to markedly attenuated Bufalin-induced cell apoptosis in T24 cells, suggesting NKA-α3 played a critical role in Bufalin-induced cell apoptosis. Taken together, the present study confirmed that Bufalin promotes tumour apoptosis and inhibits tumour growth in bladder cancer in vitro, and this antitumour effect may be ascribed to the inactivation of NKA.
Keywords: bufalin, bladder cancer, ATPase, proliferation, apoptosis, tumour
Introduction
Bladder cancer is a common malignancy and a primary cause of cancer-related morbidity and mortality in the urinary system worldwide (1). In recent years, the incidence of bladder cancer continues to rapidly increase, with approximately 75–85% of the diagnosed tumours non-muscle-invasive bladder cancer (2). With the development of detection and diagnostic techniques, most patients can be detected in the early stage and treated by the transurethral resection of tumours combined with standard intravesical chemotherapy or immunotherapy (3,4). However, 50–70% of non-muscle-invasive tumours are susceptible to recur and 10–20% of cases may rapidly progress to muscle-invasive type (5,6). Muscle-invasive bladder cancer is usually accompanied with pelvic lymph node or distant metastasis, leading to poor therapeutic effect and prognosis (7,8). Thus, intravesical chemotherapy or immunotherapy combined with surgery is thus far the most effective treatment strategy for the prevention of tumour recurrence and progress (9). Although these adjuvant drugs have exhibited relatively acceptable effects, they are always associated with multi-drug resistance and strong systemic toxicity. Therefore, exploring more effective drugs with lower toxicity will be helpful in preventing and treating the disease.
Bufalin is a major digoxin-like molecular with immunoreactivity derived from Chan Su, a traditional Chinese medicine extracted from the skin and parotid venom glands of the toad (10). It can also induce a wide range of pharmacological effects, including cardiotonic, anaesthetic, antitumour, antimicrobial, respiration-improving and so on (11,12). In particular, Bufalin has significant antitumour activity in a wide spectrum of tumour models, such as the inhibition of cell proliferation and angiogenesis, induction of cell differentiation and apoptosis, disruption of the cell cycle, reversal of multidrug resistance and modulation of the immune response (13,14). Numerous studies have indicated that NKA is a main target of Bufalin, and an aberrantly expressed NKA subunit is tightly associated with cell apoptosis and proliferation in several cancers (15,16). However, the effect of Bufalin on cell proliferation and apoptosis of bladder cancer cells has not been thoroughly clarified, and the underlying mediating mechanisms such as antitumour effects remain to be elucidated.
The present study aimed to investigate the antitumour effect of Bufalin on bladder cancer and to determine the possible molecular mechanisms of bufalin mediated by Na+-K+-ATPase (NKA), focusing on cell apoptosis and proliferation.
Materials and methods
Cell lines and cell culture
Two bladder cancer cell lines, T24 and 5637, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2.
Plasmid construction and cell transfection
Human bladder cancer cell line T24 was used to generate cells that express the NKA-α3 subunit. For construction of the vectors, the green fluorescent protein (GFP) coding sequence was inserted in the pIRES-puro vector using EcoRI and NotI digestion enzymes. The coding sequences of the α3 subunit of NKA were ligated into pIRES-puro in frame with GFP. Cell transfection was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Positive GFP-fluorescent clones were observed under a fluorescence microscope to examine GFP expression. After culture for 48 h, cells were harvested, and total RNA was extracted. Conventional RT-PCR and quantitative real-time PCR (qRT-PCR) were used to detect α3 subunit expression.
Cell proliferation assay
Cell proliferation was measured using a Cell Counting kit-8 (Beyotime Institute of Biotechnology, Jiangsu, China). After being inoculated into 96-well plates at a density of 2×103 cells/well, cells were stained with 20 µl of CCK8 reagent 48 h after transfection. Two h after incubation, cell viability was measured by detecting the absorbance of samples at 450 nm.
Cell apoptosis assay
Cell apoptosis was measured by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining (BD PharMingen, San Jose, CA, USA) following the manufacturer's instructions. In brief, T24 cells were collected in 6-well plates at a concentration of 105 cells/ml. Then, Annexin V-FITC (5 µl) and PI (5 µl) were distributed to each well, and the cells were incubated in the dark for 15 min to undergo flow cytometry (BD LSR II; BD PharMingen).
Quantitative real-time PCR
Total RNA was extracted from cancer cells by using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. After that, all RNAs were reversed transcribed into cDNA using a reverse transcription reagent kit (Takara Biotechnology, Dalian, China). Real-time quantitative PCR was performed via an Applied Biosystems SYBR-Green mix kit and the ABI 7900 Real-Time PCR system (Applied Biosystems Life Technologies, Foster City, CA, USA). Primer sequences are shown in Table I. Relative mRNA expression was normalized to GAPDH. The relative amount of mRNA was calculated using the 2−∆∆Cq method (17). All primers are shown in Table I.
Table I.
Primer sequences used in quantitative polymerase chain reaction.
| Name | Primer | Sequence 5′ to 3′ |
|---|---|---|
| Na+/K+-ATPase a1 | Forward | AGTACACGGCAGTGATCTAAAGG |
| Reverse | CAGTCACAGCCACGATAGCAC | |
| Na+/K+-ATPase a2 | Forward | GGAGATGCAAGATGCCTTTCA |
| Reverse | GCTCATCCGTGTCGAATTTGA | |
| Na+/K+-ATPase a3 | Forward | GACCTCATTTGACAAGAGTTCGC |
| Reverse | GGGCAGACTCAGACGCATC | |
| Bcl-2 | Forward | TTTGATTTCTCCTGGCTGTCT |
| Reverse | CTGATTTGACCATTTGCCTG | |
| Caspase-3 | Forward | GACAACAACGAAACCTCCG |
| Reverse | AGGGTTAGCTGCATCGACA | |
| GAPDH | Forward | ACAGCAACAGGGTGGTGGAC |
| Reverse | TTTGAGGGTGCAGCGAACTT |
Western blot analysis
Cells were collected and lysed using RIPA buffer with PMSF (Beyotime Institute of Biotechnology) on ice. Protein concentration was qualified using a bicinchoninic acid assay (BCA) kit (Beyotime Institute of Biotechnology). Equivalent amounts of protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis and subsequently transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in Tris-buffered saline (TBS) containing 5% non-fat milk. After that, membranes were incubated with primary antibody against subunit α1 (cat. no. ab7671), α3 (cat. no. ab2826; both Abcam, Cambridge, UK) and GAPDH (cat. no. 2118S; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight, followed by incubation with secondary antibodies, detected by enhanced chemiluminescent (ECL) and qualified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± SD. Differences were assessed by a two-tailed Student's t-test and one-way analysis of variance (ANOVA), and the Student-Newman-Keuls test was used as a post hoc test after ANOVA. P<0.05 was considered to indicate a statistically significant difference. All experiments were performed at least 3 times. Statistical analyses were carried out using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Bufalin inhibits cell viability and induces cell apoptosis in bladder cancer cell lines
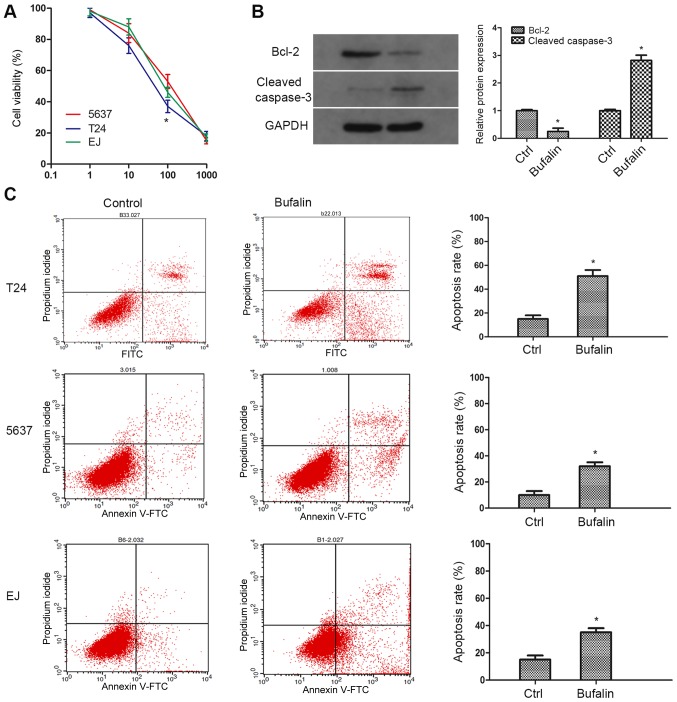
To investigate the pharmacological effect of Bufalin on bladder cancer, two bladder cancer cell lines (T24, 5637) were employed to simulate different stages of the tumour (Fig. 1). A CCK-8 assay was performed to examine sensitivities to Bufalin in tumour cells. As the result showed, compared with 5,637 cells, cell viability of T24 was significantly inhibited by Bufalin at a concentration of 100 nM (Fig. 1A). A cell apoptosis assay was performed to determine the cytostatic effect of Bufalin. As the result showed, compared with the control group, Bufalin induced remarkable apoptosis in a total of three cell lines, and the apoptosis rate in T24 cells was the most significant (Fig. 1C). Therefore, T24 cells and 100 nM concentration of Bufalin were used for subsequent research. Western blotting was additionally performed to explore the protein expression of apoptotic phenotypes caspase-3 and Bcl-2. In accordance with our expectation, the expression of caspase-3 was markedly upregulated in contrast with the expression of Bcl-2 (Fig. 1B). These results demonstrated that Bufalin inhibits tumour cell growth and promotes cell apoptosis in bladder cancer.
Figure 1.
Bufalin inhibits cell viability and induces cell apoptosis in bladder cancer cell lines. (A) Three types of bladder cancer cell lines were exposed to various concentrations of bufalin (1 to 1,000 nM) for 24 h. Cell proliferation was determined with Cell Counting kit-8 assay. (B) Western blotting was performed to examine the expression of Bcl-2 and cleaved caspase-3. (C) Annexin V-FITC/PI assay was performed to analyze cell apoptosis in T24 cells treated with Bufalin. Data are presented as means ± SD of three separated experiments (*P<0.05 vs. control group). FITC, fluorescein isothiocyanate; PI, propidium iodide.
Bufalin-induced apoptosis in bladder cancer cells through inactivation of NKA
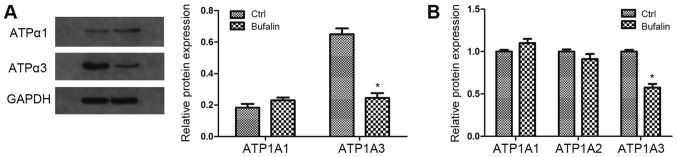
To determine whether ATPase is involved in Bufalin-induced cell apoptosis, we examined the expression of three subunits of ATPase (α1, α2 and α3) on protein or mRNA level in T24 cells. The results revealed that the expression of α3-ATPase was significantly inhibited by Bufalin on both the protein and mRNA level, while the expression of α1-ATPase and α2-ATPase was moderately changed (Fig. 2). These findings suggest that NKA is involved in the cell growth and apoptosis of bladder cancer, and the α3 subunit of ATPase may play an important role among the three subunits.
Figure 2.
Bufalin-induced apoptosis in bladder cancer cells through inactivation of Na+-K+-ATPase. (A) The protein expression of Na+/K+-ATPase α1 and α3 was detected by western blot analysis. GAPDH was used as a loading control. (B) The mRNA level of Na+-K+-ATPase α1 α2 and α3 was detected by qRT-PCR with specific primers of α1 α2 and α3 isoforms. GAPDH was used as a loading control. Data are presented as means ± SD of three independent experiments (*P<0.05 vs. control group). qRT-PCR, quantitative real-time PCR.
α3-NKA overexpression attenuated Bufalin-induced apoptosis in bladder cancer cells
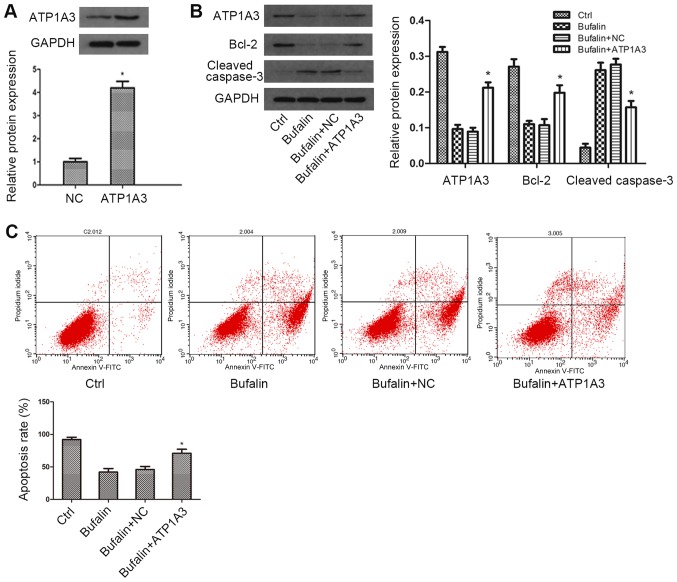
To further investigate the role of α3 subunit of NKA in Bufalin-induced cytostatic effect in bladder cancer cells, we constructed plasmids encoding α3 isoforms of ATPase to overexpress α3-ATPase. The transfection efficiency was examined by western blot analysis. As the western blot result showed, compared with the control group, the expression of α3-ATPase was remarkably upregulated on the protein level (Fig. 3A). Subsequently, we explored the expression of α3-ATPase, caspase-3 and Bcl-2 under Bufalin treatment by western blotting. As the data showed, compared with the Bufalin group, the expression of α3-ATPase was moderately increased in the α3-isoform overexpression group, while the expression change of caspase-3 and Bcl-2 induced by Bufalin was significantly weakened in α3-isoform overexpressing cells (Fig. 3B). The results from the cell apoptosis assay showed that compared with the Bufalin group, overexpression of α3-ATPase attenuated Bufalin-induced cell apoptosis (Fig. 3C). Taken together, these results confirmed that α3 subunit of NKA was the most important subunit in cell growth and apoptosis of bladder cancer.
Figure 3.
α3-Na+-K+-ATPase overexpression attenuated Bufalin-induced apoptosis in bladder cancer cells. (A) T24 cells were transfected with vectors containing sequences of α3 subunit of ATPase. Transfection efficiency was determined using western blot. (B) T24 cells overexpressing α3 isoform of ATPase were treated with Bufalin for 2 h. The protein levels of α3 isoform, Bcl-2 and cleaved caspase-3 were detected using western blot. GAPDH was used as a loading control. (C) Annexin V-FITC/PI assay was performed to examine cell apoptosis in transfected T24 cells treated with Bufalin. Data are presented as the mean ± SD of three independent experiments (*P<0.05 vs. NC group or Bufalin + NC group). FITC, fluorescein isothiocyanate; PI, propidium iodide.
Discussion
Bladder cancer is one of the most common urological malignant tumours worldwide and is the 6th leading cause of new cancer cases and the 9th leading cause of cancer-associated mortality among all types of cancer (18,19). The majority of non-muscle-invasive bladder cancers can be diagnosed and treated early for their clinical symptoms and signs; however, non-muscle-invasive bladder cancer is vulnerable to recur or progress to invasive-muscle disease, which is considered an aggressive and extremely virulent disease (20). Therefore, it is necessary to search for more effective chemotherapy drugs to improve the prognosis and survival of patients. Bufalin is a topoisomerase II inhibitor and is involved in the regulation of the development process of leukaemia, gastric, colon, breast, and ovarian cancer and other malignant tumours (21,22). Based on these results, we aimed to demonstrate that Bufalin may play an antitumour role in bladder cancer cells by downregulation of NKA.
Apoptosis is a self-killing process of programmed cell death that includes a range of cellular, morphological and biochemical changes (23). It is known that human mammalian cells exhibit two major pathways of apoptosis: The intrinsic (or mitochondrial) and extrinsic (or death receptor) signal transduction pathways (24). It has been demonstrated that the mitochondrial pathway process of apoptosis is regulated by gene expression and its activation may stimulate the degradation of cellular substrates and participate in the pathogenesis of many diseases (25). Bcl-2 may prevent the release of cytochrome c from the mitochondria to inhibit apoptosis, and caspase-3 is considered the convergence point of multiple apoptosis-activating signals that determines the extent of apoptosis. Its activation means an irreversible commitment to cellular apoptosis (26). In previous study, Qi et al (27) showed that Bufalin can reduce the expression level of Bcl-2 and stimulate the activation of caspase-3 to promote cell apoptosis through mitochondria-mediated pathways in hepatocellular carcinoma cells. One of the major features of Bufalin in the present study is the inhibition of proliferation, a vital process that plays an important role in maintaining normal tissue structure and functions (28). Recent studies showed that Bufalin induced cell apoptosis in non-small cell lung cancer, choriocarcinoma and osteosarcoma cells (29–31). To explore the effect of Bufalin on cell apoptosis and proliferation in bladder cancer, we performed an MTT assay, cell apoptosis assay and western blot. The results showed that the T24 cell line was markedly inhibited and the most sensitive to Bufalin. Moreover, Bufalin treatment resulted in the cleavage of caspase-3 activation while blocking Bcl-2 expression. These results indicated that Bufalin promotes apoptosis and inhibits proliferation in bladder cancer.
NKA is a trans-membrane protein complex in mammals, which pumps three Na+ ions out and two K+ ions into a cell per molecule of hydrolysed ATP to regulate the intracellular ion gradients (32). Apart from its function as an ion pump, NKA is also a multifunctional protein in signal transduction, cell junctions, adhesion and motility (33,34). NKA contains four isoforms of the α-subunit (α1, α2, α3 and α4) and three of the β-subunit (β1, β2 and β3) in vertebrates, and the α-subunit of NKA is the active subunit participated in the binding of cardiac steroids and NKA (16,35). Generally, the α1 subunit is widely expressed in various cell types, and the α2 subunit is mostly expressed in the heart muscle, skeletal muscle and brain. The α3 subunit is found in the central nervous system, ovaries and placental tissues, and the α4 subunit is restricted to the testes and is synthesized at the stage of spermatogonium (36,37). A number of in vivo and in vitro studies have confirmed that the α1 and α3 subunit is aberrantly expressed in a wide range of tumours. For example, the expression level of NKA-α1 is upregulated in glioblastoma, lung and skin cancers, while is downregulated in bowel cancer. On the other hand, NKA-α3 is found to be upregulated in rectal and colorectal cancers (38,39). Our results also showed that the expression level of the α3 subunit in bladder cancer cells was significantly upregulated among all α subunits. Furthermore, previous studies have demonstrated that NKA is a main target of Bufalin and is tightly associated with cell apoptosis and proliferation in malignant tumour occurrence and progression. For example, Bufalin induces apoptosis by downregulating NKA in human lymphoblastic leukaemia cells (40), and Bufalin suppresses hepatocellular carcinoma cells proliferation by negatively regulating NKA (41). In our study, we found that Bufalin significantly inhibited α3 subunit expression in T24 cells. By transfection with α3 isoform overexpressing plasmids, we found that compared with Bufalin groups, cell apoptosis was markedly attenuated in NKA-α3 overexpressing cells. These results suggested that the α3 isoform of NKA played a critical role in Bufalin-induced cell apoptosis.
In conclusion, our study indicated that Bufalin can promote cell apoptosis in bladder cancer, and this cytostatic effect may be ascribed to the inactivation of NKA. These findings implied that Bufalin has the potential to be applied as an effective antitumour medicine in the treatment of bladder cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Authors' contributions
HH was involved in the conception and design of the study, and manuscript writing. WZ was involved in the study design, supervision of all phases of the study, data collection and data analysis.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Wirth M, Plattner VE, Gabor F. Strategies to improve drug delivery in bladder cancer therapy. Expert Opin Drug Deliv. 2009;6:727–744. doi: 10.1517/17425240903022758. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Chang JK, Hou JQ, Zhao ZH, Zhang LD. Inhibition of miR-221 influences bladder cancer cell proliferation and apoptosis. Eur Rev Med Pharmacol Sci. 2017;21:3193–3199. [PubMed] [Google Scholar]
- 4.Akagashi K, Tanda H, Kato S, Ohnishi S, Nakajima H, Nanbu A, Nitta T, Koroku M, Sato Y, Hanzawa T. Recurrence pattern for superficial bladder cancer. Int J Urol. 2006;13:686–691. doi: 10.1111/j.1442-2042.2006.01386.x. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. High-risk superficial bladder cancer: Transurethral resection alone in selected patients with T1 tumor. Semin Urol Oncol. 1997;15:142–146. [PubMed] [Google Scholar]
- 6.Pasin E, Josephson DY, Mitra AP, Cote RJ, Stein JP. Superficial bladder cancer: An update on etiology, molecular development, classification, and natural history. Rev Urol. 2008;10:31–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Masson-Lecomte A, Rava M, Real FX, Hartmann A, Allory Y, Malats N. Inflammatory biomarkers and bladder cancer prognosis: A systematic review. Eur Urol. 2014;66:1078–1091. doi: 10.1016/j.eururo.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Rose TL, Deal AM, Nielsen ME, Smith AB, Milowsky MI. Sex disparities in use of chemotherapy and survival in patients with advanced bladder cancer. Cancer. 2016;122:2012–2020. doi: 10.1002/cncr.30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, Okuyama H, Nakayama M, Endo H, Nonomura N, Nishimura K, Inoue M. High-dose chemotherapeutics of intravesical chemotherapy rapidly induce mitochondrial dysfunction in bladder cancer-derived spheroids. Cancer Sci. 2015;106:69–77. doi: 10.1111/cas.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Chen C, Wang S, Zhang Y, Yin P, Gao Z, Xu J, Feng D, Zuo Q, Zhao R, Chen T. Bufalin inhibits HCT116 colon cancer cells and its orthotopic xenograft tumor in mice model through genes related to apoptotic and PTEN/AKT pathways. Gastroenterol Res Pract. 2015;2015:457193. doi: 10.1155/2015/457193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu HR, Li Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: New hope for cancer patients. Asian Pac J Cancer Prev. 2012;13:5339–5343. doi: 10.7314/APJCP.2012.13.11.5339. [DOI] [PubMed] [Google Scholar]
- 12.Yang LH, Zhang HZ, Zhang B, Chen F, Lai ZH, Xu LF, Jin XQ. Studies on the chemical constituents from the skin of Bufo bufo gargarizans Cantor. Yao Xue Xue Bao. 1992;27:679–683. (In Chinese) [PubMed] [Google Scholar]
- 13.Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J, Duan Y, Liu P, Qiu M, Li Q. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: Preparation, cellular uptake, tissue distribution, and anticancer activity. Int J Nanomedicine. 2012;7:3961–3969. doi: 10.2147/IJN.S32063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han KQ, Huang G, Gu W, Su YH, Huang XQ, Ling CQ. Anti-tumor activities and apoptosis-regulated mechanisms of bufalin on the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice. World J Gastroenterol. 2007;13:3374–3379. doi: 10.3748/wjg.v13.i24.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh CH, Wu J, Chung YY, Liu Z, Zhang RR, Chong K, Korzh V, Ting S, Oh S, Shim W, et al. Electronic supplementary material identification of a Na+/K+-ATPase inhibition-independent proarrhythmic ionic mechanisms of cardiac glycosides. Sci Rep. 2017;7:2465. doi: 10.1038/s41598-017-02496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Feng LX, Sun P, Liu W, Wu WY, Jiang BH, Yang M, Hu LH, Guo DA, Liu X. A novel bufalin derivative exhibited stronger apoptosis-inducing effect than bufalin in A549 lung cancer cells and lower acute toxicity in mice. PLoS One. 2016;11:e0159789. doi: 10.1371/journal.pone.0159789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Fan B, Zhang X, Ma Y, Zhang A. Fangchinoline induces apoptosis, autophagy and energetic impairment in bladder cancer. Cell Physiol Biochem. 2017;43:1003–1011. doi: 10.1159/000481698. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Xie L, Liu X, Zhang Y, Shen Z, Chen T, Qiu X, Sha N, Xing C, Wu Z, et al. Impact of squamous and/or glandular differentiation on recurrence and progression following transurethral resection for non-muscle invasive urothelial carcinoma of bladder. Oncol Lett. 2017;14:3522–3528. doi: 10.3892/ol.2017.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran K, Severn M. Blue light cystoscopy in patients with suspected non-muscle invasive bladder carcinoma: A review of clinical utility [Internet] Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017. [PubMed] [Google Scholar]
- 21.Kang KH, Han MH, Jeong JW, Park C, Lee SH, Lee HW, Hong SH, Choi YH, Hong SH. Bufalin sensitizes human bladder carcinoma cells to TRAIL-mediated apoptosis. Oncol Lett. 2017;14:853–859. doi: 10.3892/ol.2017.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, Xie Y, Tai Y, Gao Y, Guo W, Yu W, Li J, Feng X, Hao J, Gao Y, et al. Bufalin inhibits hTERT expression and colorectal cancer cell growth by targeting CPSF4. Cell Physiol Biochem. 2016;40:1559–1569. doi: 10.1159/000453206. [DOI] [PubMed] [Google Scholar]
- 23.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/S0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–929. doi: 10.1083/jcb.200207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang H, Yu F, Tong Z, Yuan B, Wang C. Effect of ischemia post-conditioning on skeletal muscle oxidative injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1α/β-actin mRNA, IL-6/β-actin mRNA and caveolin-3/β-actin mRNA expression in ischemia-reperfusion rabbits. Mol Biol Rep. 2013;40:507–514. doi: 10.1007/s11033-012-2087-9. [DOI] [PubMed] [Google Scholar]
- 27.Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Tang W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102:951–958. doi: 10.1111/j.1349-7006.2011.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y, Zhang Y, Luan J, Duan H, Zhang F, Yagasaki K, Zhang G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62:573–583. doi: 10.1007/s10616-010-9310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takai N, Ueda T, Ishii T, Kira N, Nishida M, Nishida Y, Nasu K, Narahara H. Effects of bufalin on the proliferation of human choriocarcinoma cells. Int J Gynecol Cancer. 2011;21:1105–1109. doi: 10.1097/IGC.0b013e318218730e. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Sha J, Zhou Y, Han K, Wang Y, Su Y, Yin X, Hu H, Yao Y. Bufalin inhibits proliferation and induces apoptosis in osteosarcoma cells by downregulating MicroRNA-221. Evid Based Complement Alternat Med. 2016;2016:7319464. doi: 10.1155/2016/7319464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren YP, Zhang MJ, Zhang T, Huang RW. Dual effects of ouabain on the regulation of proliferation and apoptosis in human umbilical vein endothelial cells: Involvement of Na(+)-K(+)-ATPase α-subunits and NF-κB. Int J Clin Exp Med. 2014;7:1214–1222. [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 34.Mobasheri A, Avila J, Cózar-Castellano I, Brownleader MD, Trevan M, Francis MJ, Lamb JF, Martín-Vasallo P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20:51–91. doi: 10.1023/A:1005580332144. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen AN, Jansson K, Sánchez G, Sharma M, Reif GA, Wallace DP, Blanco G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am J Physiol Renal Physiol. 2011;301:F897–F906. doi: 10.1152/ajprenal.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felippe Gonçalves-de-Albuquerque C, Silva Ribeiro A, da Silva Ignácio C, Castro-Faria-Neto Caire H, Burth P. Na/K pump and beyond: Na/K-ATPase as a modulator of apoptosis and autophagy. Molecules. 2017;22 doi: 10.3390/molecules22040578. pii: E578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol. 2006;290:R524–R528. doi: 10.1152/ajpregu.00838.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sakai H, Suzuki T, Maeda M, Takahashi Y, Horikawa N, Minamimura T, Tsukada K, Takeguchi N. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- 39.Mijatovic T, Ingrassia L, Facchini V, Kiss R. Na+/K+-ATPase alpha subunits as new targets in anticancer therapy. Expert Opin Ther Targets. 2008;12:1403–1417. doi: 10.1517/14728222.12.11.1403. [DOI] [PubMed] [Google Scholar]
- 40.Kawazoe N, Aiuchi T, Masuda Y, Nakajo S, Nakaya K. Induction of apoptosis by bufalin in human tumor cells is associated with a change of intracellular concentration of Na+ ions. J Biochem. 1999;126:278–286. doi: 10.1093/oxfordjournals.jbchem.a022446. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Liu X, Schwarz S, Hu L, Guo D, Gu Q, Schwarz W. Inhibitory efficacy of bufadienolides on Na+,K+-pump activity versus cell proliferation. Biochem Biophys Rep. 2016;6:158–164. doi: 10.1016/j.bbrep.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.