Abstract
The human ether-a-go-go related gene (hERG) encodes the rapid delayed rectifier K+ channel. hERG not only serves an important role in heart muscle and cardiomyocyte excitability by regulating action potential repolarization, but also represents a selective advantage for cancer cell proliferation. Arsenic trioxide is a traditional Chinese medicine, which has been previously identified as an efficient tumor suppressor, particularly in the treatment of acute pro-myelocytic leukemia. However, studies have also reported that long-term exposure to arsenicals may lead to the formation of malignant tumors. In the present study, the effect of low-dose arsenic trioxide on the proliferation and apoptosis of tumor cells was investigated, as were the potential underlying mechanisms of this effect. The data demonstrated that low-dose arsenic trioxide (0.1 µM) enhanced the viability and apoptosis of tumor cells expressing hERG channels following long-term incubation. However, in tumor cells lacking hERG channels, low-dose arsenic trioxide had no effect. Therefore, we hypothesized that this hormesis effect of low-dose arsenic trioxide on tumor cells may be associated with the hERG channel. Furthermore, low dose arsenic trioxide promoted the hERG-channel current by changing the kinetics of channel gating and prolonging the open-channel stage. Simultaneously, high-dose As2O3 (1 or 10 µM) significantly reduced the expression of hERG in tumor cells compared with the control group, which resulted in reduced proliferation rate and promotion of apoptotic rate. The results of the present study demonstrate that the dual effects of arsenic trioxide on hERG channels vary according to concentration, resulting in the dual effects on tumor cells. This provides a theoretical basis for the potential clinical application of arsenic trioxide, suggesting that hERG channels are an important target in preventing and treating tumorigenesis during arsenicosis.
Keywords: human ether-a-go-go related gene, arsenic trioxide, proliferation, apoptosis, tumorigenesis
Introduction
The human ether-a-go-go-related gene (hERG) encodes the pore-forming subunits of the rapid delayed rectifier potassium channel (1). Studies have reported that a wide variety of therapeutic compounds could induce acquired long QT syndrome (acLQTS) by inhibiting hERG channels, including antiarrhythmic drugs and antitumor agents (2–4). By contrast, the association between the enhanced activation of hERG channels and tumorigenesis remain unclear. Therefore, it is essential to examine novel compounds or therapies for restoring the physiological state of ion channel balance.
Arsenic trioxide (As2O3), a traditional Chinese medicine, is an effective treatment for acute pro-myelocytic leukemia (APL) (5,6). Previous studies have demonstrated that As2O3 has an inhibitory effect on the proliferation of various types of cancer, including APL, gastric carcinoma and breast cancer (7,8). On the other hand, the carcinogenic effects of As2O3 have been concurrently reported. It has been demonstrated that low-dose As2O3 can promote the occurrence and development of tumors (9–11). However, the underlying mechanism of this effect remains unclear. In addition, increasing evidence has indicated that plasma membrane hERG channels are aberrantly expressed in various types of cancer and serve essential roles in numerous crucial cellular events (12,13). Inhibition of the hERG channel has indicated a significant therapeutic effect in various types of tumors (12,14). Based on previous findings that As2O3 could regulate the protein expression level of hERG through multiple signal pathways (15), we hypothesize that a low dose of As2O3 exerts ahormesis effect by affecting the expression and/or function of hERG channels.
In the present study, the possible role of hERG in tumor progression was investigated when tumor cells were exposed to low-dose As2O3 (0.1 µM). Notably, the data demonstrate that a low dose of As2O3 has a hormesis effect on tumor cells, possibly due to the involvement of hERG channels. To the best of our knowledge, we are the first to hypothesize that the hERG channel participates in the dual effects of As2O3 in tumor cells. Prior to this, As2O3 was considered to be an hERG channel inhibitor (15,16) and that a low dose of As2O3, compared with a high dose of As2O3, could exert an inverse effect. Furthermore, As2O3 has been demonstrated to have a dual effect on hERG channels according to different concentrations, providing a theoretical basis for the potential clinical use of As2O3. Further research examining the crucial dosages As2O3 for apoptosis and hormesis processes is required.
Materials and methods
Cell culture
The following cell lines were purchased from Chinese Peking Union Medical College (Peking, China): 293 cells that stably expressed the wild-type hERG gene (hERG-293), the breast cancer cell line, MCF-7, and the lung cancer cell line A549. hERG-293 cells were cultured in DMEM medium containing 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 400 mg/ml of geneticin (G-418; Invitrogen; Thermo Fisher Scientific, Inc.). MCF-7 cells and A549 cells were grown in DMEM and Kaighn's Modification of Ham's F-12 (F-12K) medium (both Gibco; Thermo Fisher Scientific, Inc.) respectively, with 10% (v/v) FBS. All cells were cultured at 37°C in 5% CO2. The present study was approved by the Ethical Committee of the Harbin Medical University (Heilongjiang, China).
Whole cell patch-clamp recordings
hERG currents were recorded in the whole cell voltage-clamp (VC) mode, and action potential durations (APDs) were recorded in the current-clamp mode with an Axopatch 200B amplifier (Molecular Devices, LLC, Sunnyvale, CA, USA) in hERG-HEK293 cells. Heat-polished patch pipettes had tip resistances of 1–3 MΩ when filled with the internal pipette solution. The pipette solution for hERG current recording contained 130 mM KCl, 1 mM MgCl2·6H2O, 10 mM HEPES, 5 mM Mg-ATP, 5 mM EGTA, and 0.1 mM GTP (pH 7.3 with KOH). The extracellular solution contained 136 mM NaCl, 5.4 mM KCl, 5 mM HEPES, 1 mM MgCl2·6H2O, 1 mM CaCl2 and 10 mM glucose (pH 7.4 with NaOH). hERG currents were evoked through a 4-sec repolarizing step to −50 mV and followed by a 2-sec depolarization step with a 10 mV stepwise increase from −60 to 40 mV, with the primary holding potential being −80 mV. For the activation curves of hERG channels, cells were evoked through a 2.5-sec repolarizing step to an +40 mV inactivation step, following a 10 mV stepwise increase from −120 to +20 mV, while the onset of inactivation curves was recorded with a 10 mV stepwise increase from −120 to +30 mV. The time interval of onset of inactivation was investigated by fitting a single exponential function to current traces in the third pulse of the protocol. The recovery of hERG channels from inactivation curves was recorded with a 10 mV stepwise increase from −120 to +30 mV. The time constant of recovery from inactivation was calculated by fitting a single exponential function to the peak of tail current traces between −60 and −30 mV. Experiments were recorded and analyzed on one cell per recording. Currents were fitted to a Boltzmann function using Clampfit 9.2 (Axon Instruments; Molecular Devices, LLC, Sunnyvale, CA, USA), where V1/2 is the half-maximum activation voltage.
Western blot analysis
The protein expression level of hERG was monitored using western blot analysis. As2O3 was diluted (0.1, 1 and 10 µM) and added to 1×106 hERG-293 cells for 24 h at 37°C prior to analysis by western blotting. The cells were washed using ice-cold PBS. Subsequently, protein was harvested with radio immunoprecipitation assay buffer containing 1% protease inhibitor (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein concentration was assessed using the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China). A total of 150 µg protein was loaded per lane and separated using 8% SDS-PAGE. Proteins were then transferred to nitrocellulose membranes (Agilent Technologies, Inc., Santa Clara, CA, USA) and incubated with 5% non-fat milk (BD Biosciences, Franklin Lakes, NJ or San Jose, CA, USA) for 2 h at room temperature. The membranes were subsequently incubated with primary antibodies against hERG (dilution, 1:1,000; cat. no., sc-20130; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin (dilution, 1:500; cat. no., TA-09; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) overnight at 4°C with agitation. The membranes were washed with 0.05% Tris-buffered saline (TBST) three times and incubated with IRDye 800CW-labelled, goat anti-rabbit IgG secondary antibodies (1:8,000; cat. no. 926–32211; Li-Cor Inc., Lincoln, NE, USA) for 1 h in the dark at room temperature. Subsequent to being washed three times with 0.05% TBST, bands were detected and analyzed with the Odyssey CLx Infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). To quantify the western blotting data, the intensities of proteins of interest in each gel were firstly normalized to their respective actin intensities, then the normalized intensities were compared with the intensity of the control group and expressed as relative values to their controls.
MTT assay
Proliferation was assessed using an MTT assay. Briefly, 5×103 cells were treated with various concentrations (0.1, 1 and 10 µM) of As2O3 for 24 and 72 h. Next, a mixture of 180 µl culture medium and 20 µl MTT reagent (Sigma-Aldrich; Merck KGaA) was added to each well. Subsequently, 100 µl DMSO was added to each well and mixed to solubilize the colored crystals. The absorbance was measured at 490 nm after 4 h of incubation at 37°C.
Flow cytometry (FCM) analysis of apoptosis
Quantitative assessment of apoptosis was conducted using an Annexin V Assay kit (Beyotime Institute of Biotechnology). Cells were centrifuged at 1,000 × g for 5 min at 4°C following trypsinization, then washed with ice-cold PBS twice. The pellet was resuspended in the ice-cold binding buffer provided with the kit. Subsequently, 10 µl Annexin V-FITC and 5 µl propidium iodide (PI) were added to the cell suspension, which was maintained on ice in the dark for 15 min. The samples were then assessed for viable (Annexin V−/PI−), early apoptotic (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic (Annexin V−/PI+) cells using a flow cytometer (Fc500MDL) and analyzed by CXP Analysis Software (version 2.0) (both Beckman Coulter, Inc., Brea, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of the mean. The Student's t-test was used for comparisons between two groups, and analysis of variance was used for the comparison of multiple groups with the Student-Newman-Keuls post hoc test. P<0.05 (two-tailed) was considered to indicate a statistically significant difference. GraphPad Prism 5.0 software was used for statistical analysis (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Effects of hormesis on tumor cells induced by As2O3
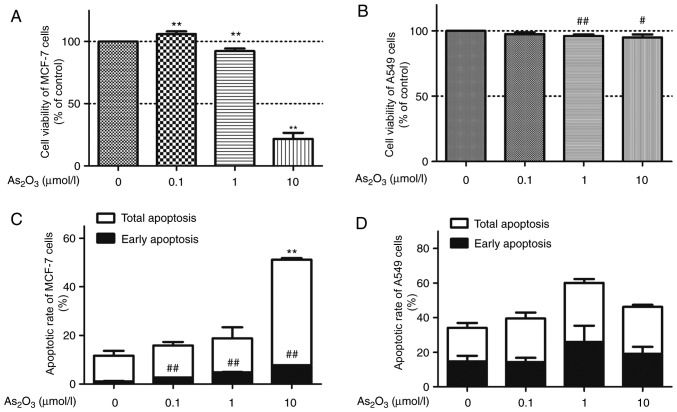
The viability in response to As2O3 stimulation was compared in two types of cancer cells: MCF-7 and A549 cells. In our previous screening experiments, it was indicated that MCF-7 cells overexpressed hERG channels, whereas A549 cells rarely expressed hERG channels. In contrast with the A549 cells, the MCF-7 cells expressed endogenous hERG channels (17). An MTT assay was performed to assess viability following treating the cells with 0.1, 1 and 10 µM of As2O3 for 72 h (Fig. 1A). Application of 1 or 10 µM As2O3 produced a suppressive effect on MCF-7 cell proliferation, resulting in a 7.69 and 78.30% reduction in MCF-7 cell viability at 1 and 10 µM, respectively. Notably, a dual effect of As2O3 was observed, since MCF-7 cell viability increased by ~6% in the 0.1 µM As2O3 group compared with the control group. However, a low concentration (0.1 µM) of As2O3 did not express the hormesis effect on the proliferation of A549 cells. A high dose of As2O3 (1 and 10 µM) reduced the viability of A549 cells in a concentration-dependent manner, while 0.1 µM As2O3 did not significantly affect A549 proliferation compared with the control group (Fig. 1B).
Figure 1.
Cell viability and apoptotic rate of MCF-7 and A549 cells was examined with 0, 0.1, 1 and 10 µM As2O3 treatment. (A) Viability of MCF-7 cells was determined by MTT assay. (B) Viability of A549 cells was determined by MTT assay. (C) Apoptotic rate of MCF-7 cells was determined by flow cytometry. (D) Apoptotic rate of A549 cells was determined by flow cytometry. Data are presented as the mean ± standard deviation (n=3). #P<0.05 vs. 0 µM As2O3, **P<0.01 vs. 0 µM total apoptosis or, ##P<0.01 vs. 0 µM early apoptosis. As2O3, arsenic trioxide.
Considering that numerous antitumor drugs suppress proliferation and simultaneously promote apoptosis, a quantification of As2O3-induced apoptotic cells was conducted via flow cytometry with Annexin V/PI analysis. MCF-7 cells and A549 cells were treated with 0.1, 1 and 10 µM of As2O3 for 72 h. As2O3 treatment resulted in a dose-dependent increase in apoptotic rate in MCF-7 cells compared with control (Fig. 1C). Subsequent to treatment with 0.1, 1 and 10 µM As2O3 for 72 h, apoptotic rate of 15.9, 18.8 and 51.1% in MCF-7 cells were observed, respectively. However, As2O3 did not indicate significant cytotoxic effects in A549 cells (Fig. 1D; P>0.05).
In the apoptosis experiments, all cells were cultured without FBS for 72 h, therefore only apoptosis occurred. However, in the proliferation experiments, all the cells were cultured with FBS for 72 h, resulting in proliferation and apoptosis. Therefore, the data primarily demonstrated that a low dose of As2O3 could stimulate MCF-7 proliferation, whereas no significant effect on A549 cells was exhibited.
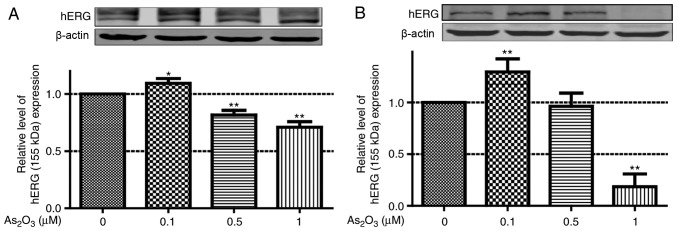
Dual effects of As2O3 on the expression of hERG channels
In our previous study, it was demonstrated that 3 µM As2O3 could damage the hERG current and downregulate the expression of hERG channels by disturbing the trafficking process of hERG protein to the cellular membrane (18). However, the effect of low-dose As2O3 on hERG channels requires further investigation. Therefore, in the present study, western blot analysis was used to identify the effect of low-dose As2O3 on the protein expression level of hERG. Following incubation with low-dose As2O3 (0.1, 0.5 and 1 µM) for 24 h, hERG protein expression level in hERG-293 cells was examined. Doses of 0.5 and 1 µM As2O3 significantly reduced hERG protein expression. By contrast, 0.1 µM As2O3 increased hERG protein expression (Fig. 2A). Subsequently, the endogenous hERG levels were also determined in MCF-7 cells, as well as following treatment with 0.1, 1 and 10 µM As2O3 for 24 h (Fig. 2B). The protein expression level of hERG in the 0.1 µM As2O3 group increased by 35.17% compared with the control group.
Figure 2.
Western blot analysis and quantification of protein expression. (A) Relative expression levels of hERG protein in hERG-293 cells with 0, 0.1, 1 and 10 µM As2O3 treatment. (B) Relative expression levels of hERG protein in MCF-7 cells with 0, 0.1, 1 and 10 µM As2O3 treatment. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05 or **P<0.01 vs. control group. As2O3, arsenic trioxide; hERG, human ether-a-go-go related gene.
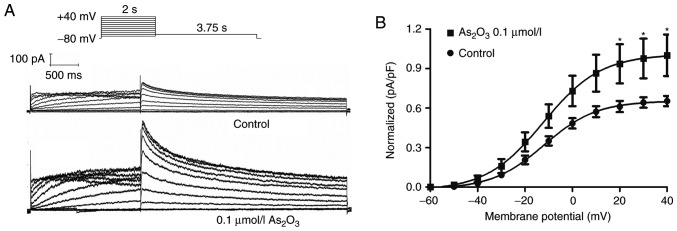
Low-dose As2O3 enhances the expression of functional hERG channels
Since the transport speed of ions through the membrane depends on the number of functional ion channel proteins, it is necessary to detect the current (Ikr or IhERG) to verify whether 0.1 µM As2O3 could enhance the expression of functional hERG channels. Patch clamp recordings were conducted to detect the effect of 0.1 µM As2O3 on hERG-293 cells after 24 h of incubation. IhERG was elicited during 2-sec depolarizations (ranging from −60 to +40 mV) from a holding potential of −80 mV, then a repolarization to −50 mV was used to elicit the tail current (Fig. 3A). It was demonstrated that 0.1 µM As2O3 enhanced the hERG tail current amplitude by 53.14% (Fig. 3B). These results suggest that 0.1 µM As2O3 significantly upregulated the expression of functional hERG channels.
Figure 3.
Effect of low-dose As2O3 on hERG tail current. (A) Voltage-clamp protocol and representative hERG current traces recorded from a hERG-293 cell incubated with As2O3 for 24 h. (B) Statistical analysis of hERG tail currents. Data are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control. As2O3, arsenic trioxide; hERG, human ether-a-go-go related gene; VC, voltage-clamp, pa/pf, picoamperes per picofarad.
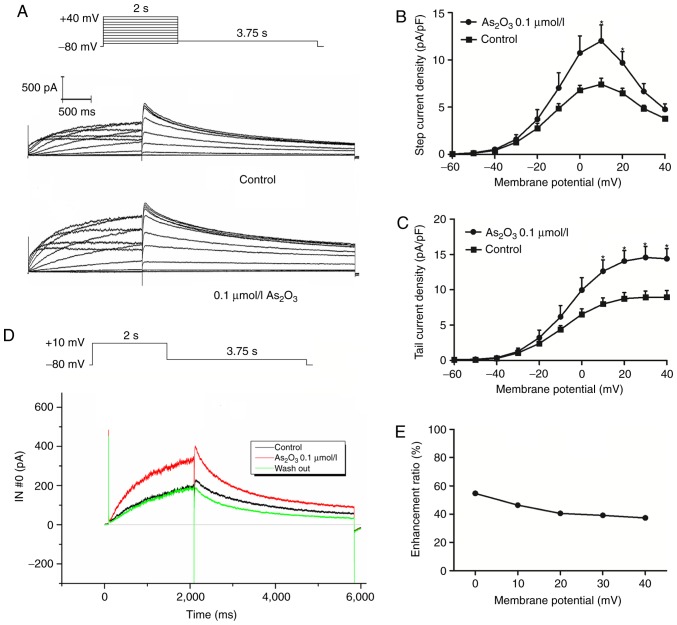
Low-dose As2O3 accelerates hERG channel activation
The properties and/or kinetics of potassium channels are often altered by drugs. Therefore, the activation, steady-state inactivation, and the time courses of the hERG channel were determined in the control and the As2O3-treated groups. The transient effect of 0.1 µM As2O3 on the hERG current was measured. To investigate the effect of low-dose As2O3 (0.1 µM) on hERG potassium channels, patch-clamp experiments were performed on 293 cells stably expressing the wild-type hERG gene. The current-voltage (I–V) association is indicated in Fig. 4. Whole-cell current was first recorded under control conditions, then 0.1 µM As2O3 was added through perfusion equipment at 1.5 ml/min for 5 min prior to the next recording. In the presence of 0.1 µM As2O3, the hERG current was notably increased and could be subsequently reversed subsequent to washing out As2O3. The activation current and tail current reached peak amplitudes at 10 and 20 mV, respectively. The ratio of enhancement was calculated using the activation current from 0 to 20 mV and tail current from 0 to 40 mV with the function: (Iato-Ictl)/Ictl. These electrophysiological results suggested that 0.1 µM As2O3 had a significant effect (P<0.05) on the activation of hERG channels.
Figure 4.
Current-voltage association of hERG channels in the control and As2O3-treated groups. (A) Voltage-clamp protocol and representative hERG current traces recorded from a hERG-293 cell under low-dose As2O3 acute perfusion. (B) Current density of hERG channels. (C) Statistical analysis of hERG tail current. (D) Representative hERG current traces recorded from a hERG-293 cell under low-dose As2O3 acute perfusion and subsequent wash out. (E) Enhancement ratio of hERG current under different membrane potentials. Data are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group. As2O3, arsenic trioxide; VC, voltage-clamp; hERG, human ether-a-go-go related gene; pa/pf, picoamperes per picofarad; I–V, current-voltage; IN#0, channel 0 of Axopatch 200B Amplifier.
Low-dose As2O3 treatment affects hERG channel kinetics
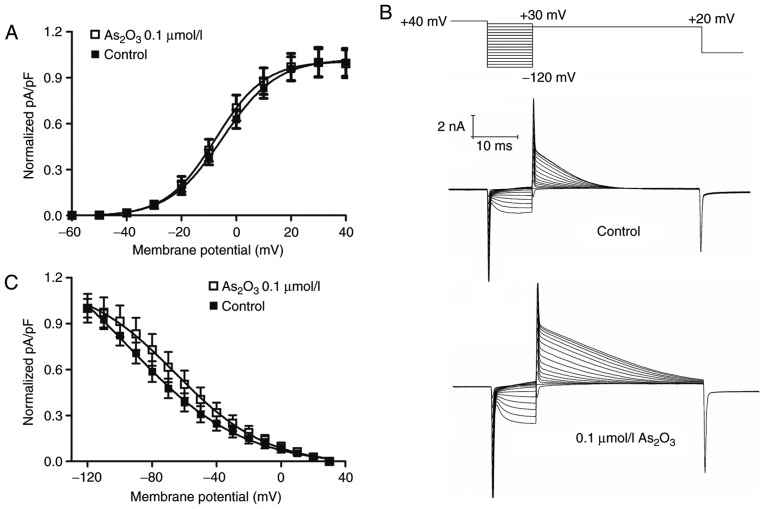
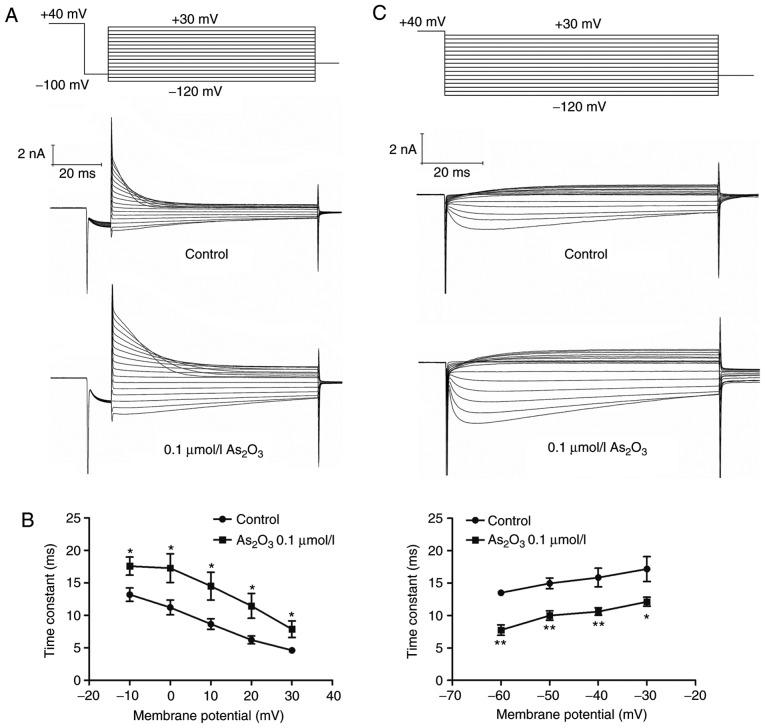
In a previous study, it was reported that various drugs can influence ion channels by altering the voltage dependence or kinetics of channel gating (19). To investigate the underlying mechanism of the enhanced hERG currents induced by low dose As2O3 with transient administration, its effects on the voltage dependence of activation and inactivation were examined (Fig. 5). To obtain the half maximal voltage, a Boltzmann function was fitted to the data. The results indicated that As2O3 shifted the half maximal activation voltage of hERG channels slightly towards negative potentials, from −4.95±0.26 mV (in the control group) to −7.50±0.25 mV (Fig. 5A) and half maximal inactivation voltage to more positive potentials, from −94.64±5.25 mV (in the control group) to −65.58±1.71 mV (Fig. 5C). The time interval of onset of inactivation was investigated by fitting a single exponential function to current traces in the third pulse of the protocol (Fig. 6A). The statistical data (Fig. 6B) indicated that inactivation was slower in the presence of As2O3. The result indicated that low-dose As2O3 significantly altered the hERG channel kinetics by decreasing the time period of recovery from inactivation (Fig. 6C).
Figure 5.
Effects of low dose As2O3 on voltage-dependent activation curves and inactivation curves of hERG channels. (A) Voltage-dependent activation curves for the control and following exposure to low-dose As2O3 for 24 h. (B) Voltage-clamp protocol and representative current tracing for steady-state inactivation. (C) Inactivation curve of hERG channels after treatment with low-dose As2O3 for 24 h. Data are presented as the mean ± standard error of the mean (n=6). As2O3, arsenic trioxide; hERG, human ether-a-go-go related gene; VC, voltage-clamp; pa/pf, picoamperes per picofarad.
Figure 6.
Effects on voltage dependence of activation and inactivation. (A) Voltage-clamp protocol and representative current tracing for the onset of inactivation. (B) Effect of low-dose As2O3 on the time period of onset of inactivation following treatment for 24 h. (C) Voltage-clamp protocol and representative current tracing for recovery from inactivation. (D) Effect of low-dose As2O3 on the recovery from inactivation following treatment for 24 h. Data are presented as the mean ± standard error of the mean (n=6). *P<0.05 or **P<0.01 vs. control group. As2O3, arsenic trioxide; VC, voltage-clamp.
Discussion
As2O3 is a popular therapeutic agent for various types of cancer and has been demonstrated to have a high therapeutic effect in APL. However, the adverse effects of arsenic trioxide, including cardiotoxicity and carcinogenicity, remain a clinical problem (20). The adverse effects of chronic arsenic toxicity in humans are referred to as arsenicosis (21,22). Although researchers have reported significant associations between the occurrence of tumors and arsenicosis, the underlying mechanism remains unclear.
The most common oxidation states of arsenic include arsenite and arsenate (23). Among these, As2O3 is the most prevalent inorganic arsenical found in the air or in underground water (AsO2−) (24). Therefore, the present study was mainly based on As2O3 and not arsenate. In our previous studies, As2O3 was demonstrated to regulate the expression of hERG channels (18). In addition, it has been demonstrated that the proliferation-facilitating effect of As2O3 is significantly more pronounced in hERG-expressing cells than in hERG-lacking tumor cells (25), which indicates that hERG channels serve an important role in the regulation of tumor progression. Accordingly, we hypothesized that hERG channels may be involved in the process of arsenicosis. To investigate this, viability and apoptotic rate was compared in response to As2O3 stimulation in MCF-7 and A549 cells. The results indicate that a medium or high dose of As2O3 inhibited proliferation and promoted apoptosis. However, a low dose of As2O3 treatment simultaneously promoted proliferation and increased apoptosis in MCF-7 cells compared with control group. This hormesis phenomenon is similar to that of the tumor necrosis factor (TNF) superfamily. TNF has been demonstrated to be an endogenous cancer promoter that stimulates cancer cell invasion and proliferation (22,23), but by contrast, TNF has also been proven to be an endogenous cancer killer (26). This hormesis phenomenon was not observed in A549 cells. This suggests that MCF-7 cells were more sensitive than A549 cells to As2O3.
The hormesis of As2O3 has also been demonstrated by other research groups (10,25). It was reported that a low dose of As2O3 could stimulate osteoblast and MTLn3 cell proliferation (10). Wang et al (27) demonstrated that hERG protein expression is associated with tumor-cell proliferation and cancer development. To address whether hERG channels were involved in this process, 293 cells overexpressing hERG channels were used in the present study. hERG current and electrophysiological characteristics are relatively easy to detect in this cell model (28–30). hERG-293 cells were incubated with 0.1, 0.5 and 1 µM As2O3 for 24 h. The results demonstrated that 0.1 µM As2O3 was able to increase the expression of hERG channels compared with the control group. In addition, 0.1 µM As2O3 increased endogenous hERG expression in MCF-7 cells. This is notable since it was previously accepted that As2O3 was a hERG channel inhibitor. This is the first study, to the best of our knowledge, to demonstrate that a low dose of As2O3 could exert an inverse effect compared with high-dose administration.
To verify the results, the influence of a low dose of As2O3 on hERG current and channel kinetics was analysed. The results indicated that a low dose of As2O3 not only increased the hERG current, but also accelerated hERG channel activation. In addition, low-dose As2O3 treatment markedly affected hERG channel kinetics by causing a slower rate of channel inactivation and faster recovery time, compared with the untreated cells.
The hERG channel has previously been demonstrated to positively regulate tumor proliferation (31). The data of the present study revealed that a low dose of As2O3 promoted hERG protein expression and provided a plausible explanation for arsenicosis-induced tumorigenesis. However, the present study did not investigate the involvement of any other molecules or signaling pathways, which could affect this process. Therefore, further research is required.
It should be noted that the research of the present study also has certain limitations. Due to the specificity of tumor cells and limitations in experimental techniques, particularly the patch-clamp technique, tracking and recording the characteristics of ion channels on tumor cells was not possible. Therefore, hERG-293 cells were used as a mature cell model for the investigation of electrophysiological characteristics of hERG potassium channels, as previously described (18,32).
In summary, the present study provides evidence that a low dose of As2O3 promotes tumorigenesis through upregulating hERG channel expression and affecting hERG channel kinetics. Although As2O3 was previously demonstrated to be an hERG channel inhibitor, it was demonstrated that a low dose of As2O3 exhibits the opposite effect, promoting hERG channel expression and enhancing hERG current. These findings may help to identify therapeutic strategies for patients with arsenicosis, and provide a novel understanding of the association between As2O3 and hERG channels.
Acknowledgements
The authors would like to thank Dr Yuanqi Shi (Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China) for providing assistance with the patch-clamp technology.
Funding
This study was supported by grants from the Major Program of the National Natural Science Foundation of Heilongjiang (grant no. ZD 2015015), and the National Natural Science Foundation of China (grant nos. 81673636 and 81173050).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ is responsible for the writing and design of the manuscript. DY is responsible for performance of the western blot assay. HG is responsible for performance of the western blot assay. FL is responsible for performance of the Patch clamp. LL is responsible for performance of Flow Cytometry. LZ is responsible for performance of the Patch clamp. CY is responsible for the design of the subject and writing of the manuscript. BL is the provider of the funding and is responsible for the study design.
Ethics approval and consent to participate
There is no clinical, patient, tissue or animal experimentation involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 2.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, Shah R. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: Clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21:1216–1231. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 3.Farkas AS, Nattel S. Minimizing repolarization-related proarrhythmic risk in drug development and clinical practice. Drugs. 2010;70:573–603. doi: 10.2165/11535230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi LL, Ma Q, Wang SQ. Treatment of 32 cases with recurring acute promyelocytic leukemia with tablets of composite natural indigo. Zhonghua Er Ke Za Zhi. 2005;43:702–703. (In Chinese) [PubMed] [Google Scholar]
- 6.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 7.Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999;35:1258–1263. doi: 10.1016/S0959-8049(99)00106-9. [DOI] [PubMed] [Google Scholar]
- 8.Ye J, Li A, Liu Q, Wang X, Zhou J. Inhibition of mitogen-activated protein kinase kinase enhances apoptosis induced by arsenic trioxide in human breast cancer MCF-7 cells. Clin Exp Pharmacol Physiol. 2005;32:1042–1048. doi: 10.1111/j.1440-1681.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Chen X, Wu J, Liu H, Ji Z, Shi H, Gao C, Han D, Wang L, Liu Y, et al. Low-dose arsenic trioxide enhances 5-aminolevulinic acid-induced PpIX accumulation and efficacy of photodynamic therapy in human glioma. J Photochem Photobiol B. 2013;127:61–67. doi: 10.1016/j.jphotobiol.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Raja WK, Satti J, Liu G, Castracane J. Dose response of MTLn3 cells to serial dilutions of arsenic trioxide and ionizing radiation. Dose Response. 2013;11:29–40. doi: 10.2203/dose-response.11-025.Raja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapathy S, Li P, Fagman J, Yu T, Lafontant J, Zhang G, Chen C. Low doses of arsenic, via perturbing p53, promotes tumorigenesis. Toxicol Appl Pharmacol. 2016;306:98–104. doi: 10.1016/j.taap.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Becchetti A, Crescioli S, Zanieri F, Petroni G, Mercatelli R, Coppola S, Gasparoli L, D'Amico M, Pillozzi S, Crociani O, et al. The conformational state of hERG1 channels determines integrin association, downstream signaling, and cancer progression. Sci Signal. 2017;10 doi: 10.1126/scisignal.aaf3236. pii: eaaf3236. [DOI] [PubMed] [Google Scholar]
- 13.Crociani O, Lastraioli E, Boni L, Pillozzi S, Romoli MR, D'Amico M, Stefanini M, Crescioli S, Masi A, Taddei A, et al. hERG1 channels regulate VEGF-A secretion in human gastric cancer: Clinicopathological correlations and therapeutical implications. Clin Cancer Res. 2014;20:1502–1512. doi: 10.1158/1078-0432.CCR-13-2633. [DOI] [PubMed] [Google Scholar]
- 14.Fortunato A. The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells. Cell Oncol (Dordr) 2017;40:367–378. doi: 10.1007/s13402-017-0328-6. [DOI] [PubMed] [Google Scholar]
- 15.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Wan X, Dennis AT, Obejero-Paz C, Overholt JL, Heredia-Moya J, Kirk KL, Ficker E. Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome. J Biol Chem. 2011;286:2843–2852. doi: 10.1074/jbc.M110.125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SZ, Jiang M, Zhen YS. Correlation of HERG K+ channel protein expression to chemosensitivity of tumor cells to doxorubicin and its modulation by erythromycin. Ai Zheng. 2005;24:924–929. (In Chinese) [PubMed] [Google Scholar]
- 18.Zhang Y, Dong Z, Jin L, Zhang K, Zhao X, Fu J, Gong Y, Sun M, Yang B, Li B. Arsenic trioxide-induced hERG K(+) channel deficiency can be rescued by matrine and oxymatrine through up-regulating transcription factor Sp1 expression. Biochem Pharmacol. 2013;85:59–68. doi: 10.1016/j.bcp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson GM, Blanche T, Mansfield K, Tran Y. Differential blockade of neuronal voltage-gated Na(+) and K(+) channels by antidepressant drugs. Eur J Pharmacol. 2002;452:35–48. doi: 10.1016/S0014-2999(02)02239-2. [DOI] [PubMed] [Google Scholar]
- 20.Pace C, Dagda R, Angermann J. Antioxidants protect against arsenic induced mitochondrial Cardio-toxicity. Toxics. 2017;5 doi: 10.3390/toxics5040038. pii: E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar A, Paul B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere. 2016;158:37–49. doi: 10.1016/j.chemosphere.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Oremland RS, Stolz JF. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 2005;13:45–49. doi: 10.1016/j.tim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Orloff K, Mistry K, Metcalf S. Biomonitoring for environmental exposures to arsenic. J Toxicol Environ Health B Crit Rev. 2009;12:509–524. doi: 10.1080/10937400903358934. [DOI] [PubMed] [Google Scholar]
- 24.Reeve BB, Burke LB, Chiang YP, Clauser SB, Colpe LJ, Elias JW, Fleishman J, Hohmann AA, Johnson-Taylor WL, Lawrence W, et al. Enhancing measurement in health outcomes research supported by Agencies within the US Department of Health and Human Services. Qual Life Res. 2007;16(Suppl 1):S175–S186. doi: 10.1007/s11136-007-9190-8. [DOI] [PubMed] [Google Scholar]
- 25.Xu WX, Liu Y, Liu SZ, Zhang Y, Qiao GF, Yan J. Arsenic trioxide exerts a double effect on osteoblast growth in vitro. Environ Toxicol Pharmacol. 2014;38:412–419. doi: 10.1016/j.etap.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, Chen Q, Huang C, Xu X. CYLD promotes TNF-α-induced cell necrosis mediated by RIP-1 in human lung cancer cells. Mediators Inflamm. 2016;2016:1542786. doi: 10.1155/2016/1542786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- 28.Li P, Ninomiya H, Kurata Y, Kato M, Miake J, Yamamoto Y, Igawa O, Nakai A, Higaki K, Toyoda F, et al. Reciprocal control of hERG stability by Hsp70 and Hsc70 with implication for restoration of LQT2 mutant stability. Circ Res. 2011;108:458–468. doi: 10.1161/CIRCRESAHA.110.227835. [DOI] [PubMed] [Google Scholar]
- 29.Dong ZX, Zhao X, Gu DF, Shi YQ, Zhang J, Hu XX, Hu MQ, Yang BF, Li BX. Comparative effects of liensinine and neferine on the human ether-a-go-go-related gene potassium channel and pharmacological activity analysis. Cell Physiol Biochem. 2012;29:431–442. doi: 10.1159/000338497. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Prinsen JK, Bersell KR, Shen W, Yermalitskaya L, Sidorova T, Luis PB, Hall L, Zhang W, Du L, et al. Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.115.003560. pii: e003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staudacher I, Jehle J, Staudacher K, Pledl HW, Lemke D, Schweizer PA, Becker R, Katus HA, Thomas D. HERG K+ channel-dependent apoptosis and cell cycle arrest in human glioblastoma cells. PLoS One. 2014;9:e88164. doi: 10.1371/journal.pone.0088164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi YQ, Yan M, Liu LR, Zhang X, Wang X, Geng HZ, Zhao X, Li BX. High glucose represses hERG K+ channel expression through trafficking inhibition. Cell Physiol Biochem. 2015;37:284–296. doi: 10.1159/000430353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.