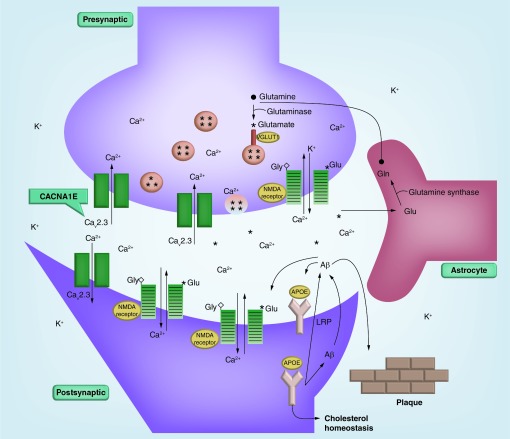
Figure 1 . The schematic of the neurometabolic cascade initiated by the mechanical disruption of cellular membranes and axonal stretching shows proteins responsible for plasticity and repair of neurons (APOE, LPR), ion channels (NMDA receptor, Cav2.3), and a neuromediator transporter (VGLUT1).
Persistent action potentials resulting from a neuron stretch release glutamate (shown as asterisks [*]) and other excitatory amino acids. Glutamate binds to the NR2 subunits within the NMDA receptors. This binding activates NMDA receptors, and initiates Ca2+ influx. Glutamate binding to NMDA receptor also leads to the efflux of K+ ions. Glutamate uptake into neurosecretory vesicles (shown as circles with asterisks) is facilitated by multiple glutamate transporter proteins including VGLUT1. In astrocytes (surrounding, supportive glial cells), glutamate is converted to inactive glutamine (•) by glutamine synthase, released to the extracellular compartment, and eventually reconverted to glutamate inside neurons in the course of the glutamine-glutamate cycle. Ca2+ enters the cells predominantly through the NMDA channels (striped rectangles), and voltage-activated Ca2+ channels (open rectangles), e.g., Cav2.3 encoded by CACNA1E gene. In response to injury, the synthesis of APOE is rapidly induced through the unique mechanism controlled by alternative splicing. The accumulating APOE participates in lipid transport and membrane repair. APOE binds to low-density lipoprotein receptor (LRP), and alters metabolism of amyloid-β (Aβ) peptides. Different APOE isoforms may affect intracellular trafficking of the Aβ peptide, and facilitate plaque buildup.