Abstract
Sorafenib has been the only approved systemic therapy for hepatocellular carcinoma until very recently. However, the radiologic assessment of its biological activity is a disputed matter as at least five different criteria have been proposed. In this review, we describe the characteristic of the Response Evaluation Criteria In Solid Tumors (RECIST), European Association for the Study of The Liver (EASL), modified RECIST (mRECIST), Response Evaluation Criteria In the Cancer of the Liver (RECICL) and Choi criteria. The existing comparative studies are reported together with recent pieces of evidence, analyzing the reasons behind the split between recommendations of the scientific societies and regulatory agencies. Future perspectives in the wake of the impending results of the immunotherapy trials are also discussed.
KEYWORDS : Choi, hepatocellular carcinoma, immunotherapy, mRECIST, nivolumab, RECICL, RECIST, regorafenib, sorafenib
Graphical abstract
Practice points.
The most used radiologic criteria of response are based either on the measurement of tumor whole (RECIST 1.1) or of its viable portion (mRECIST).
Criteria based on viable tumor measurement discriminate a subgroup of partial responders patients apparently characterized by a better prognosis compared with those with stable disease.
All of the criteria are equally able to discriminate progressors and nonprogressors patients, which is the most relevant parameter in clinical practice.
Regardless of the adopted criteria, imaging-derived surrogate parameters (such as overall response rate, progression-free survival and time to radiologic progression) remain imperfectly predictive of overall survival in patients treated with systemic therapies.
‘Progressive disease’ concept includes different patterns of progression characterized by different prognosis.
The performance of the currently proposed criteria is based solely on experiences with anti-VEGFR drugs. The performance with different drugs (should they become available) is unknown.
Decisions in clinical practice, as well as evaluations of the results of Phase II trials, should not be rigidly based on surrogate end points alone.
In parallel, in clinical practice decisions about drug withdrawal should be solely based on the assessment of progressive disease.
The performance of the currently proposed criteria should be validated with different drugs: important information about this point will come from the immunotherapy trials.
The improvement of the overall survival (OS) is the main objective of any anticancer treatment. However, surrogate end points are needed in clinical practice. In addition, the assessment of OS may be time-consuming in Phase II clinical trials, slowing the development of drugs potentially able to improve the prognosis of patients lacking therapeutic alternatives.
Generally, the containment of tumor growth is the key strategy to prolong OS. In fact, tumor expansion and spread are the most common causes of the deterioration of patient's general conditions, exacerbation of comorbidities and death in neoplastic patients. These aspects are particularly relevant in the case of hepatocellular carcinoma (HCC). In most cases, this malignancy occurs and disseminates in cirrhotic livers [1], fatally accelerating the natural course of the chronic liver failure.
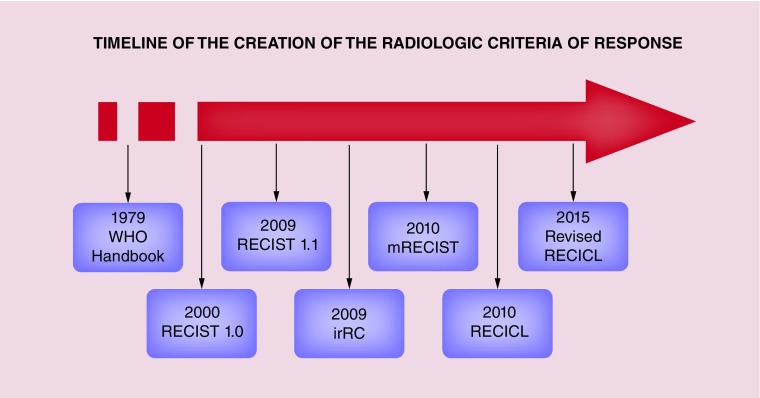
On this basis, an accurate measure of tumor mass by imaging techniques has always been a hot topic, especially to identify of surrogate end points of OS. The first attempts date back to the 1960s, with the first major step being reached years later with the publication of the 1979 WHO Handbook [2]. The subsequent tumultuous development of imaging techniques led to the creation of the Response Evaluation Criteria in the Solid Tumors (RECIST) working group and to the development of the respective criteria in 2000 [3].
The RECIST 1.0 has been a cornerstone in the scientific history of imaging. All of the currently proposed criteria use the structure of RECIST 1.0 as a common backbone, even if differing one from the others in relevant points. In this review we briefly describe the most used radiological response criteria in the evaluation of systemic treatments for HCC, illustrating the major open problems and the possible future scenarios.
Radiologic criteria of response
• General features
Deriving from RECIST 1.0 (and by extension from the 1979 WHO handbook), all of the currently proposed criteria retain common features. For example, baseline imaging assessment includes target lesions and nontarget lesions. Target lesions are the most representative measurable lesions of the entire tumor burden. On the other hand, nontarget lesions include the remaining measurable lesions and any nonmeasurable lesion (for instance macrovascular invasion, malignant ascites, etc.) [3].
Overall response is established: accurately quantifying dimensional changes of target lesions; providing an approximate estimate of dimensional changes of nontarget lesions; verifying the occurrence of new lesions (this event automatically defining a neoplastic progression). The overall response is categorized into four groups, maintaining the original WHO recommendations. These groups are: complete response (CR; complete disappearance of neoplastic lesions), partial response (PR; basically the dimensional reduction of target lesions below a predetermined threshold), progressive disease (PD; dimensional increase in target lesions above an established threshold, unambiguous dimensional increase in nontarget lesions or occurrence of new lesions) and stable disease (SD; variations that do not fall under criteria defining PR or PD) [2,3].
The differences between the different proposed criteria mainly regard the methods adopted in measuring target lesions (total tumor vs arterialized tumor, unidimensional vs bidimensional), and in setting the thresholds defining PR and SD (Table 1).
Table 1. . Comparative view of the main characteristics of currently used and/or proposed criteria for the assessment of the radiologic response of hepatocellular carcinoma to sorafenib.
| Parameters | Measurements of lesions | Evaluated parameter(s) | Max number of target lesions – total | Max number of target lesions – per organ | Definition of PR | Definition of PD | Lymph nodes | Criteria for defining new liver lesions |
|---|---|---|---|---|---|---|---|---|
| RECIST 1.1 | Unidimensional | Total dimensions | 5 | 2 | ≥30% decrease in the sum of diameters of target lesions† | ≥20% increase in the sum of diameters of target lesions† | Considered as target lesions if short axis ≥15 mm | Unequivocal appearance |
| EASL | Bidimensional | Enhanced tumor | 5 | 2 | ≥30% decrease in the sum of diameters of enhancing target lesions† | ≥20% in the sum of the diameters of enhancing target lesions‡ | Considered as target lesions if short axis ≥15 mm | Unequivocal appearance and typical HCC pattern |
| MRECIST | Unidimensional | Enhanced tumor | 5 | 2 | ≥30% decrease in the sum of diameters of enhancing target lesions† | ≥20% in the sum of the diameters of enhancing target lesions‡ | Porta hepatis lymph nodes: short axis ≥20 mm, all other locations ≥15 mm | Unequivocal appearance and typical HCC pattern |
| RECICL | Bidimensional | Total dimensions, enhanced tumor | 5 | 2 (3 for liver) | Tumor necrosis of 50–100% or 50–100% reduction in tumor size | Tumor enlargement of ≥50% (excluding the area of necrosis after treatment) | Considered as target lesions if short axis ≥15 mm | Unequivocal appearance and typical HCC pattern |
| CHOI | Undimensional | Total dimension, tumor density | 5 | 2 | Decrease in tumor size >10% or a decrease in tumor density >15% | Increase in tumor size >20% without post-treatment hypodense change | Considered as target lesions if short axis ≥15 mm | Unequivocal appearance |
†Taking as reference the baseline sum of diameters.
‡Taking as reference the smallest sum of diameters on study.
EASL: European Association for the Study of the Liver; HCC: Hepatocellular carcinoma; mRECIST: Modified RECIST; PD: Progressive disease; PR: Partial response; RECICL: Response Evaluation Criteria in the Cancer of the Liver; RECIST: Response Evaluation Criteria in Solid Tumor.
• Total tumor assessment (RECIST 1.1)
Directly deriving from the original RECIST 1.0, RECIST 1.1 are the most used criteria in the evaluation of the response to anticancer therapies in the majority of solid tumors. These criteria have been developed on the basis of the pieces of evidence acquired using conventional cytotoxic agents, which are able to induce a tumor shrinking [4].
RECIST 1.1 evaluates the response of up to five target lesions, with up to two organ lesions. The maximum diameter of each target lesion is measured, with the exception of the lymph nodes lesions (for which the short axis is evaluated, provided that it reaches a minimum threshold of 15 mm). The maximum diameter can include areas of intralesional necrosis [4].
RECIST 1.1 remains the only universally accepted criteria by regulatory agents in clinical trials for many different cancers, including HCC [5].
• Viable tumor assessment (EASL, mRECIST, RECICL)
RECIST shows some drawbacks when applied to the specific field of HCC. A wide range of local and regional treatments can be used to treat this malignancy, most of which can induce permanent areas of necrosis of the treated lesions. By literally applying the RECIST, treatment-induced necrosis areas would be interpreted as SD or PD, even in the actual absence of any residual disease [6].
This problem prompted the European Association for the Study of the Liver (EASL) to develop its own criteria for measuring the viable (i.e., arterialized) portions of the treated nodules. A 2D evaluation was also recommended in these criteria. Further, only nodules exhibiting the typical characteristics of arterial wash-in followed by wash-out could be considered as new lesions [6]. This last recommendation addressed the peculiar characteristic of the cirrhotic liver, in which a wide range of non-neoplastic lesions (such as large regenerative nodules) can develop in the follow-up.
In 2010, Lencioni and Llovet [7] embraced these recommendations proposing the modified RECIST (mRECIST). These criteria endorsed most of the suggestions of the EASL expert panel, differing mainly in suggesting a 1D evaluation of the lesions. Some other confounding factors specifically related to liver cirrhosis were also addressed (porta hepatis lymph nodes, ascites) [7].
According to the mRECIST, the largest diameter of the arterialized portion of a maximum of five target lesions (maximum two per organ) should be measured. The threshold defining PR and PD is similar to those defined by the RECIST [7]. Currently, mRECIST are the recommend criteria to assess response to sorafenib according to the EASL guidelines [8].
The Response Evaluation Criteria in the Cancer of Liver (RECICL) has been proposed by the Japanese Society of Hepatology [9]. These criteria are based on the measurement of the viable portion of the tumor as well. However, some significant differences with the previously described criteria have to be considered: the evaluation is bidirectional and not unidirectional, similarly to the original EASL recommendations [6]; tumor viability is evaluated in all contrastographic phases and not only during the arterial phase; evaluation of nonarterialized lesions is also performed and, in case of a significant growth over time, they can define PD (thus addressing the possibility of hypovascular HCCs); tumor burden is estimated by the sum of the products of the two major diameters of the target lesions, consequently modifying the cut-offs that define PR and SD [9].
• Other proposed criteria
A different perspective is provided by the measurement of the tumor density. In 2007, Choi et al. demonstrated that the combined assessment of both tumor size and density was able to provide important prognostic information in patients with metastatic gastrointestinal stromal tumors treated with imatinib, a tyrosine kinase inhibitor [10].
Based on the similar biological effects of imatinib and sorafenib in terms of reduction of vascular cancer feeding, some studies evaluated the performance of the Choi criteria in the setting of advanced HCC, with interesting preliminary results [11–13].
Finally, some authors underlined the need for a volumetric assessment of tumor modifications to increase the accuracy and the reproducibility of imaging even in HCC patients [13–15]. Even if not currently used in clinical practice, a volumetric assessment might become easier and more common following the recent widespread availability of new automatic and semiautomatic software [13–15].
Which set of criteria is best to assess response to sorafenib?
The performance of the response criteria is usually evaluated on their ability to reflect the biological effects induced by antineoplastic treatments and, more importantly, in predicting the OS. When assessing the effects of the locoregional treatments for HCC, evidence unequivocally suggests that the criteria based on viable tumor measurement (in particular mRECIST) are superior to criteria based on total tumor measurement. This superiority is due to the inability of the latter in discriminating the areas of treatment-induced necrosis from the viable tumor [16–19].
When it comes to systemic therapies, however, the topic becomes far more debated. Sorafenib was the first approved systemic drug for HCC following the results of the Phase III SHARP trial, in which it proved to be superior to placebo in improving the OS of patients with HCC not amenable for locoregional therapies [20]. Sorafenib also improved the progression-free survival (PFS) and time to radiologic progression (TTP), indirectly suggesting their suitability as surrogate end points [20]. Of note, targeted drugs such as sorafenib reduce the tumor volume in a small percentage of patients. Nonetheless, they are able to induce a stabilization of the neoplastic disease [21]. A ‘vascular shutdown’ (i.e., a reduced arterialization) of the tumor nodules is one of their therapeutic actions [20]. When assessed by imaging techniques, this shutdown may be optimistically exchanged for ‘necrosis’ [22].
Consequently, it seemed logical to hypothesize that criteria based on viable tumor measurement could better reflect the therapeutic effects of sorafenib and better predict the OS. A series of retrospective comparative studies [11–13,23–26] tried to verify this hypothesis (Table 2).
Table 2. . Retrospective studies comparing the performance of different radiologic criteria of response to sorafenib for the treatment of hepatocellular carcinoma.
| Patients (n) | Analyzed criteria | First imaging follow-up | Main result | Other results | Ref. |
|---|---|---|---|---|---|
| 60 | RECIST, mRECIST, CHOI, EASL | 2.1 months (range 1.0–6.2) | OS correlated only with response according to Choi criteria | – | [12] |
| 190 | RECIST, mRECIST | ≤4 weeks | OS correlated with objective response (CR + PR) defined according to mRECIST but not according to RECIST 1.1 | – | [26] |
| 22 | RECIST, mRECIST, CHOI, EASL, TV | 8 ± 2 weeks | OS correlated only with response according to TV | – | [13] |
| 64 | RECIST, mRECIST, CHOI, EASL | 2.1 months (range 1.4–3.0) | OS associated with objective response (CR + PR) defined according to Choi but not according to the other criteria | – | [11] |
| 156 | RECIST, mRECIST, RECICL | 4–6 weeks | OS correlated only with categorization according to the RECICL | In patients categorized as SD or PD by RECIST1.1, only reclassification by RECICL was associated with OS | [25] |
| 48 | RECIST, mRECIST, EASL | 4–6 weeks | Significant OS differences between PD and SD for all criteria, between PR and SD only for mRECIST and EASL | – | [24] |
| 53 | RECIST, mRECIST | 4 and 8 weeks | Both methods provided good correlation with OS | In patients classified as SD according to RECIST, response according to mRECIST identifies a different prognosis | [23] |
A particular attention was given to the correlation between overall survival (OS) and categorization of response according to each set of criteria.
EASL: European Association for the Study of the Liver; HCC: Hepatocellular carcinoma; mRECIST: Modified RECIST; PD: Progressive disease; PR: Partial response; RECICL: Response Evaluation Criteria in the Cancer of the Liver; RECIST: Response Evaluation Criteria in Solid Tumor; TV: Total volume measurement.
Edeline et al. [23] demonstrated that the mRECIST are able to identify a subgroup of PR patients with a particularly favorable prognosis. However, both RECIST and mRECIST correlated with the OS to the log-rank test. These aspects were subsequently confirmed by Ogasawara et al. [24]. In other studies, however, different set of criteria were associated with superior prognostic abilities, including RECICL, Choi and total volume assessment [11–13,25,26].
Taken together, these studies do not offer an easy interpretation because of the heterogeneity of their design as well as some intrinsic limitations. In particular, the main difficulties arise from their retrospective nature and from the heterogeneous and sometimes extremely early timing of the response assessment. Further, some studies considered patients with stable disease as ‘nonresponders’ to sorafenib, further complicating their interpretation [26].
With these limitations in mind, criteria evaluating tumor arterialization (mRECIST, RECICL) or density (Choi) actually seem to be able to identify a subgroup of PR patients, otherwise classified as patients with SD by the RECIST. Even if interesting, this information has not a meaningful impact in clinical practice since progression (and not response) guides the therapeutic decision. In the absence of significant adverse events both PR and SD patients continue the ongoing therapy. Conversely, PD patients may benefit from switching to second-line therapy or entering a clinical trial. In this regard, the ability of the different criteria in discriminating progressors from patients with disease control appeared to be substantially overlapping [11–13,23–26]. This is confirmed by recent evidence. First, a meta-analysis of two Phase II clinical trials comparing nintedanib versus sorafenib showed that response according to RECIST 1.0 and mRECIST equally predicted OS [27]. Second, in the recent successful RESORCE trial, no significant differences in the assessment of TTP were found using RECIST 1.1 or mRECIST [28].
In conclusion, criteria based on viable tumor/tumor density measurement may be better in the identification of partial responders but are equally able to identify progressors compared with the RECIST. As such, no definite judgment about the superiority of these methods can be reached at the moment.
Open problems
In the field of systemic treatments for HCC surrogate end points such as PFS and TTP are unreliably associated with the OS, regardless of the adopted imaging criteria. This has been seen in several Phase III trials in which drugs could improve these end points without prolonging OS. It has been confirmed by the preliminary data of the REFLECT trial [3]. This noninferiority randomized clinical trial compared lenvatinib versus sorafenib as a frontline systemic treatment for unresectable HCC. Lenvatinib was superior to sorafenib in terms of secondary end points such as PFS (7.4 vs 3.7 months, hazard ratio 0.66 [95% CI: 0.57–0.77; p < .00001]), TTP (8.9 vs 3.7 months, hazard ratio 0.63 [95% CI: 0.53–0.73; p < .00001]) and overall response rate (24.1 vs 9.2%, odds ratio 3.13 [95% CI: 2.15–4.56; p < .00001]). This overwhelming superiority in secondary end points did not translate into a meaningful survival benefit, even if the primary end point of noninferior OS was met (13.6 vs 12.3 months, hazard ratio 0.92 [95% CI: 0.79–1.06]) [29].
The reason for the imperfect correlation between surrogate end point and OS likely relies on the basis that not all patterns of progressions are equal in terms of prognostic implications. In a brilliant paper, Reig et al. demonstrated that the appearance of new extrahepatic lesions has a far worse prognostic impact than the enlargement of pre-existing lesions or the appearance of new intrahepatic nodules [30]. Thus, a careful evaluation of the progression pattern is indeed required in clinical practice before switching to a second line treatment. Also, it must be considered in the design of clinical trials to avoid major flaws in both design and outcome.
Reproducibility between different radiology operators is another open problem which has been relatively under-investigated. Among the papers comparing the predictivity of the imaging criteria, only two works evaluated this aspect. Ronot et al. [11] found a moderate agreement for Choi criteria (k = 0.58) and a substantial agreement both for RECIST 1.1 (k = 0.65) and mRECIST (k = 0.67). Bargellini et al. [13] found a slightly higher rate of concordance (RECIST 1.1: k = 0.83; mRECIST: k = 0.85). However, the experience of radiology operators also plays a role in maintaining such high rates of concordance, as in clinical practice imaging is not always read in tertiary centers. A comprehensive analysis of the sources of discordance is therefore of paramount importance [22]. For instance, it is well known that the changes induced by sorafenib are not homogenous across the tumor sites and that this problem may reduce the reproducibility in the response assessment [31]. Moreover, it has also been shown that expert operators may choose different target lesions at the baseline examinations, adding a further element of variability [32].
As a final problem, all of the currently available information about the performance of the radiologic criteria of response in systemic treatments for HCC derive from studies investigating a single drug (i.e., the VEGFR inhibitor sorafenib). For almost 10 years, sorafenib has been the only registered systemic drug for HCC. In the next future, two other VEGFR inhibitors (regorafenib and lenvatinib) will be used in clinical practice. Also, more and more data will become available from the immunotherapy trials [33]. The latter event will be of particular interest as these drugs can lead to a peculiar kind of radiologic response. First, differently from antiangiogenic drugs, they seem able to obtain a tumor shrinkage and thus their biological benefit is not merely related to the delay of progression. Second, a ‘pseudoprogression’ due to a lymphocytic infiltration and inflammation of tumor nodules may be also noted [34,35]. These phenomena probably justify the surprising results of seminal trials of ipilimumab in melanoma, in which about 40% of patients who obtained a meaningful OS benefit had received an initial response of PD according to the RECIST 1.1 [36,37]. To address this issue, new radiologic criteria of response specific for immunotherapy drugs have been proposed (immune-related response criteria – irRC). The most relevant and innovative feature of these criteria is that new lesions occurring at the first follow-up do not automatically define PD and need to be confirmed at a follow-up imaging assessment [38].
Conclusion
Many different radiologic criteria of response have been proposed for HCC. Criteria based on viable tumor measurement (such as mRECIST) are superior to those based on whole-tumor measurement (i.e., RECIST 1.1) in the assessment of locoregional treatments, however, their putative superiority in assessing the response to systemic treatments is far more dubious. In particular, RECIST 1.1 and mRECIST equally identify progressors and do not differ in the assessment of surrogate end points such as PFS and TTP. As such, regulatory agencies still require RECIST 1.1 as criteria of choice in most HCC systemic trials. Further, the difficulties of surrogate end points in predicting OS is another open problem which characterize the specific field of HCC and that deserve further research endeavors.
Future perspective
Some of the aformentioned problems will not receive an answer in brief times, in particular modification of the of rules of the regulatory agencies are not expected soon. As for the difficulties of the radiologic criteria of response in predicting the OS, a number of different solutions may be of help in the future. First, the use of more complex prognostic tools including both clinical and radiology data. Second, a centralization of liver imagining in a limited number of centers with radiologists who have received a long-term dedicated training may improve the reproducibility and the accuracy of the radiological evaluation. While already performed in the setting of some clinical trials, centralization might be associated with logistical difficulties and increased time in medical reporting if proposed in the real-life clinical practice. Third, the aforementioned widespread diffusion of automatic and semi-automatic systems may be of help in assessing the total tumor volume with both accuracy and reproducibility. Measurement of the whole tumoral burden instead of a limited number target lesions may prove of help in selected cases and better reflect the within-patient neoplastic genetic heterogeneity (which potentially lead to a different response of the different clones to the antineoplastic treatments).
It is not easy to predict whether these innovations will take place in a temporal landscape of 5–10 years and if they will actually improve the reliability of the surrogate end points of OS. Until then, therapeutic decisions in clinical practice, as well as the decision to progress from Phase II to Phase III clinical trials, should not be strictly and uncritically based on radiologic assessment alone.
On the contrary, in the next 5 years novelties may come from the immunotherapy trials. In other malignancies, checkpoint inhibitors not only represented a terrific therapeutic innovation but also led to the creation of specifically designed response criteria due to their mechanism of action. The results of the immunotherapy trials may validate (or not) the performance of the currently used criteria in the setting of drugs with different mechanism of action.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. International Agency for Research on Cancer; Lyon, France: 2012. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11.http://globocan.iarc.fr [Google Scholar]
- 2.World Health Organization. WHO; Geneva, Switzerland: 1979. WHO handbook for reporting results of cancer treatment. [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(22):228–234. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]; • Most used radiologic criteria of response in oncology.
- 5.Edeline J, Palmer D, Blanc JF, et al. mRECIST for systemic therapies: more evidence is required before recommendations can be made. J. Hepatol. 2017;67(1):195. doi: 10.1016/j.jhep.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, et al. EASL panel of experts on HCC European Association for the Study of the Liver: clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J. Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]; • Radiologic criteria of response currently endorsed by the European Association for the Study of the Liver (EASL).
- 8.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Ueshima K, Kubo S, et al. Response evaluation criteria in cancer of the liver (RECICL) (2015 revised version) Hepatol. Res. 2016;46(1):3–9. doi: 10.1111/hepr.12542. [DOI] [PubMed] [Google Scholar]
- 10.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 2007;25(13):1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 11.Ronot M, Bouattour M, Wassermann J, et al. Alternative response criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19(4):394–402. doi: 10.1634/theoncologist.2013-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavanier M, Ayav A, Sellal C, et al. CT imaging findings in patients with advanced hepatocellular carcinoma treated with sorafenib: alternative response criteria (Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumor (mRECIST)) versus RECIST 1.1. Eur. J. Radiol. 2016;85(1):103–112. doi: 10.1016/j.ejrad.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Bargellini I, Scionti A, Mismas V, et al. Identification of responders to sorafenib in hepatocellular carcinoma: is tumor volume measurement the way forward? Oncology. 2014;86(4):191–198. doi: 10.1159/000358599. [DOI] [PubMed] [Google Scholar]; • Description of a possible widespread use of tumor volume measurement.
- 14.Monsky WL, Garza AS, Kim I, et al. Treatment planning and volumetric response assessment for yttrium-90 radioembolization: semiautomated determination of liver volume and volume of tumor necrosis in patients with hepatic malignancy. Cardiovasc. Intervent. Radiol. 2011;34(2):306–318. doi: 10.1007/s00270-010-9938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M, Pellerin O, Bhagat N, et al. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2012;23(12):1629–1637. doi: 10.1016/j.jvir.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prajapati HJ, Spivey JR, Hanish SI, et al. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE) Ann. Oncol. 2013;24(4):965–973. doi: 10.1093/annonc/mds605. [DOI] [PubMed] [Google Scholar]
- 17.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J. Hepatol. 2011;55(6):1309–1316. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Vincenzi B, Di Maio M, Silletta M, et al. Prognostic relevance of objective response according to EASL Criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS ONE. 2015;10(7):e0133488. doi: 10.1371/journal.pone.0133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262(2):708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 21.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four Phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Reig M, Sangro B. Assessment of treatment efficacy in hepatocellular carcinoma: response rate, delay in progression or none of them. J. Hepatol. 2017;66(6):1114–1117. doi: 10.1016/j.jhep.2017.02.032. [DOI] [PubMed] [Google Scholar]; •• Editorial summarizing the current debate on RECIST 1.1 versus mRECIST in the evaluation of systemic treatments for hepatocellular carcinoma.
- 23.Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by response evaluation criteria in solid tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118(1):147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara S, Kanai F, Ooka Y, et al. Initial response to sorafenib by using enhancement criteria in patients with hepatocellular carcinoma. Hepatol. Int. 2013;7(2):703–713. doi: 10.1007/s12072-013-9425-4. [DOI] [PubMed] [Google Scholar]
- 25.Arizumi T, Ueshima K, Takeda H, et al. Comparison of systems for assessment of post-therapeutic response to sorafenib for hepatocellular carcinoma. J. Gastroenterol. 2014;49(12):1578–1587. doi: 10.1007/s00535-014-0936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada J, Hidaka H, Nakazawa T, et al. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res. Notes. 2015;8:609. doi: 10.1186/s13104-015-1565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer T, Palmer DH, Cheng AL, et al. mRECIST to predict survival in advanced hepatocellular carcinoma: analysis of two randomised Phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017;37(7):1047–1055. doi: 10.1111/liv.13359. [DOI] [PubMed] [Google Scholar]
- 28.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet. 2016;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AL, Finn RS, Qin S, et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2017;S35:4001. [Google Scholar]
- 30.Reig M, Rimola J, Torres F, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58(6):2023–2031. doi: 10.1002/hep.26586. [DOI] [PubMed] [Google Scholar]; •• Demonstration of the different prognosis of different progression patterns.
- 31.Reig M, Darnell A, Forner A, et al. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin. Liver Dis. 2014;34(4):444–455. doi: 10.1055/s-0034-1394143. [DOI] [PubMed] [Google Scholar]
- 32.Tovoli F, Renzulli M, Mulazzani L, et al. 2017. Predicting outcome in patients with intermediate or advanced hepatocellular carcinoma receiving sorafenib: influence of the radiologist experience on the prognostic value of the different proposed radiologic criteria of response. Presented at. 2–5 February. [Google Scholar]
- 33.Tovoli F, De Lorenzo S, Barbera MA, et al. Postsorafenib systemic treatments for hepatocellular carcinoma: questions and opportunities after the regorafenib trial. Future Oncol. 2017;13(21):1893–1905. doi: 10.2217/fon-2017-0166. [DOI] [PubMed] [Google Scholar]
- 34.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA. 2008;105(8):3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat. Clin. Pract. Oncol. 2008;5(9):557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, Phase II, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 37.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm Phase II study. Ann. Oncol. 2010;21(8):1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 38.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]; • New immune-related response criteria used in the assessment of response to immunotherapeutic drugs in oncology.


