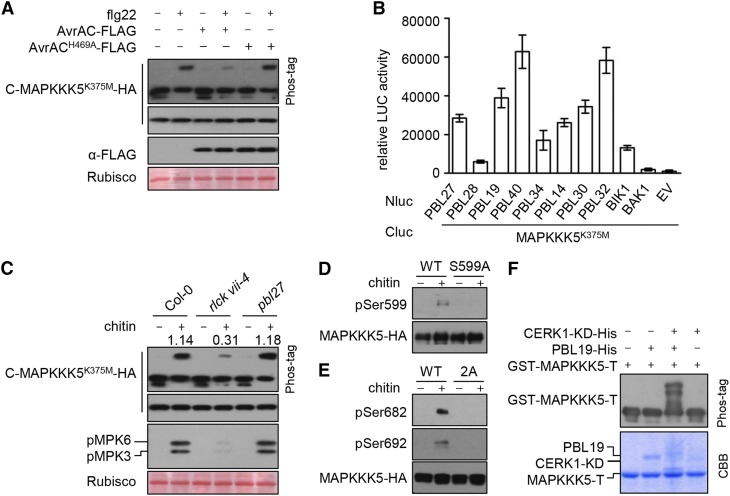
Figure 4.
RLCK VII Subfamily Members Link PRRs to MAPKKK5.
(A) AvrAC inhibits flg22-induced phosphorylation of MAPKKK5 C terminus. C-MAPKKK5K375M-HA was coexpressed with AvrAC or AvrACH469A in Col-0 protoplasts, treated with flg22 for 10 min, and total protein was subjected to SDS-PAGE containing Phos-tag acrylamide and detected by immunoblotting with anti-HA antibody.
(B) MAPKKK5K375M interacts with selected members of RLCK VII. The indicated Nluc and Cluc constructs were transiently expressed in N. benthamiana plants for luciferase complementation assays. Error bars indicate sd.
(C) Chitin-triggered phosphorylation of MAPKKK5 C terminus and MPK3/6 is impaired in rlck vii-4 sextuple mutant, but occurs normally in pbl27. Protoplasts expressing C-MAPKKK5K375M-HA were treated with chitin. Total protein was subjected to SDS-PAGE containing Phos-tag acrylamide and detected by immunoblotting with anti-HA antibody for MAPKKK5 phosphorylation and anti-pERK antibody for MPK3/6 phosphorylation. Numbers indicate relative band density of pMPK3/6 normalized to the loading control (Rubisco).
(D) and (E) Chitin-triggered phosphorylation of MAPKKK5 at Ser-599, Ser-682, and Ser-692. Stable transgenic seedlings expressing MAPKKK5-HA (WT), MAPKKK5S599A-HA (S599A), and MAPKKK5S682/692A-HA (2A) were sprayed with chitin. The proteins were affinity purified with anti-HA antibodies and detected by immunoblot analysis with anti-pSer599 (D), anti-pSer682 (E), and anti-pSer692 (E) antibodies to investigate MAPKKK5 phosphorylation.
(F) In the presence of the CERK1 kinase domain (CERK1-KD), PBL19 phosphorylates MAPKKK5 C-terminal tail in vitro. GST-MAPKKK5-T was incubated with PBL19-His and/or CERK1-KD-His proteins, and total protein was subjected to SDS-PAGE containing Phos-tag acrylamide and detected by immunoblot analysis with anti-GST antibodies. The smeared appearance of CERK1-KD and PBL19 in lanes 3 and 4 of the Coomassie Brilliant Blue (CBB)-stained gel likely reflects phosphorylation of these proteins.