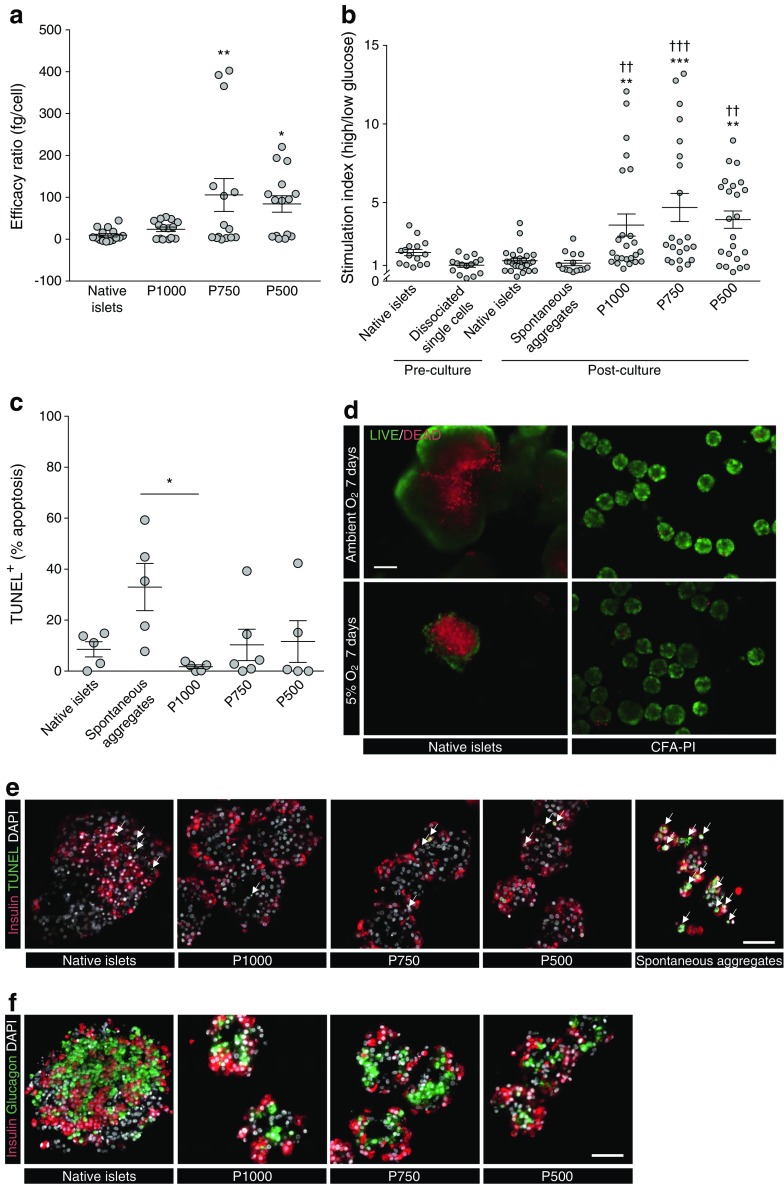
Fig. 2.
(a) Overall CFA-PI showed enhanced GSIS per input cell, and the efficacy ratio for P750 and P500 pseudoislets was significantly improved compared with native islets: 105.70 ± 39.24 fg/cell and 84.19 ± 19.79 fg/cell vs 9.53 ± 3.87 fg/cell; *p < 0.05, **p < 0.01; n = 15 per group, one-way ANOVA with Dunnett’s correction for multiple comparisons. (b) Stimulation index (insulin secretion under high glucose divided by secretion under low glucose) was assessed for islets upon receipt and immediately following dissociation to single cells, and after 3–5 days of culture as islets, spontaneous aggregates and CFA-PI. CFA-PI showed significant improvements over both native islets (**p < 0.01, ***p < 0.001) and spontaneous aggregates (††p < 0.01, †††p < 0.001); data presented as mean ± SEM, n ≥ 12 per group, Kruskal–Wallis test with Dunn’s correction for multiple comparisons. (c) TUNEL staining after 3–5 days in culture showed significantly increased apoptosis in the spontaneous aggregates compared with the P1000 CFA-PI (33.0 ± 9.2% vs 1.8 ± 0.7%; *p < 0.05; n = 5, Kruskal–Wallis test), other comparisons were not statistically significant. (d) Representative images show differences in cell death in native islets and CFA-PI cultured for 7 days under ambient and hypoxic (5% O2) atmospheric conditions and stained for live (green) and dead (red) cells (scale bar, 100 μm). (e) Immunostaining of cultured native islets, CFA-PI (P1000, P750 and P500) and spontaneous aggregates prior to transplant for insulin (red), TUNEL+ (green/arrows) and nuclei (DAPI, grey) shows the increased apoptosis in spontaneous aggregates. (f) Immunolocalisation of insulin (red) and glucagon (green) shows potential structural differences in the location of alpha and beta cells between native islets (intermingled) and CFA-PI (beta cells at periphery) (scale bars, 50 μm)