Abstract
Abstract
Key Message Powdery mildew resistance in two strawberry mapping populations is controlled by both stable and transient novel QTL of moderate effect. Some low transferability of QTL across wider germplasm was observed.
Abstract
The obligate biotrophic fungus Podosphaera aphanis is the causative agent of powdery mildew on cultivated strawberry (Fragaria × ananassa). Genotypes from two bi-parental mapping populations ‘Emily’ × ‘Fenella’ and ‘Redgauntlet’ × ‘Hapil’ were phenotyped for powdery mildew disease severity in a series of field trials. Here, we report multiple QTL associated with resistance to powdery mildew, identified in ten phenotyping events conducted across different years and locations. Six QTL show a level of stable resistance across multiple phenotyping events; however, many other QTL were represented in a single phenotyping event and therefore must be considered transient. Subsequent screening of identified QTL across a validation set determined whether identified QTL remained closely linked to the associated resistance gene in the wider germplasm. Furthermore, a preliminary association analysis identified a novel conserved locus for further investigation. Our data suggest that resistance is highly complex and that multiple, primarily additive, sources of quantitative resistance to powdery mildew exist across strawberry germplasm. Utilisation of the reported markers in marker-assisted breeding or genomic selection would lead to improved powdery mildew-resistant strawberry cultivars, particularly where the studied parents, progeny and close pedigree material are included in breeding germplasm.
Electronic supplementary material
The online version of this article (10.1007/s00122-018-3128-0) contains supplementary material, which is available to authorized users.
Introduction
Podosphaera aphanis (Wallr.) U. Braun & S. Takam. (syn. Sphaerotheca macularis, P. macularis) is a global pathogen on strawberry (Fragaria × ananassa) (Peries 1962), where late season infestations in untreated fields result in unmarketable fruit and severe yield loss (Nelson et al. 1995). Powdery mildew was rated the most important disease by large UK strawberry producers, with 65% of UK growers reporting common outbreaks of P. aphanis (Calleja 2011). The transfer of strawberry field production into protected systems has been associated with a heightened incidence of powdery mildew, and greater fungal biomass has been observed on strawberry fruits in polytunnel environments (Xiao et al. 2001).
P. aphanis infects the leaves, fruit, stolon and flowers of strawberry plants (Paulus 1990). A higher disease level on the abaxial (lower) leaf surface is due to infection of emergent susceptible leaves before unfolding, with ontogenic resistance developing in the adaxial surface prior to exposure (Asalf et al. 2014). For conidia on host tissue, optimum conditions for germination and colony establishment can lead to disease symptom development within 4 days, upon which conidiation begins anew (Amsalem et al. 2006). Although a high relative humidity (RH) is required for germination and release of conidia (Amsalem et al. 2006), conidial germination is inhibited by free water (Peries 1962). The sexual ascospores overwintering on dead plant foliage are considered to be a major source of infection in early spring; as such, the removal of old strawberry foliage as a source of inoculum should reduce epidemics (Xu et al. 2008a).
P. aphanis is considered to have a small host range with host specificity of strawberry and raspberry (Harvey and Xu 2010). Across the powdery mildews, it is understood that many host-specific adaptations have arisen through convergent evolution, with over 400 different fungal species causing powdery mildew on 9838 different angiosperm hosts (Amano 1986; Braun 1987; Mori et al. 2000).
For strawberries, powdery mildew is primarily controlled using fungicide application as many varieties have poor levels of disease resistance. Fungicides with modes of action targeting fungal respiration, nucleic acid synthesis, sterol biosynthesis and signal transduction are commonly used to control P. aphanis on strawberry (Lainsbury 2016). However, the evolution of resistance to sterol demethylation inhibitor fungicides has posed challenges for P. aphanis control (Sombardier et al. 2010). Such challenges have been exacerbated by the loss of active ingredients associated with stricter European regulations (e.g. 91/414/EEC; Colla et al. 2012), highlighting a greater requirement for P. aphanis-resistant breeding resources.
Previous studies have shown high variation in powdery mildew resistance within strawberry breeding germplasm and high heritability of resistance, indicating the large potential for enhancing disease resistance through breeding (Nelson et al. 1995). Utilisation of pre-breeding data and marker-assisted or genomic selection (Whitaker et al. 2012) will aid the production of durable powdery mildew resistance and reduce growers reliance on fungicide control.
Materials and methods
Disease phenotyping
Plant material was created through a cross between the powdery mildew-resistant strawberry cultivar ‘Emily’ and the susceptible cultivar ‘Fenella’ to produce the ExF mapping population of 181 individuals which segregates for mildew resistance. This was phenotyped over 4 years in six locations denoted: 2011, 2012a, 2012b, 2013a, 2013b and 2014. All phenotyping events were conducted at East Malling Research, Kent, UK (now NIAB EMR), except 2013b which was conducted in Paraje Moriteja, Rociana del Condado, Spain. Both 2012a and 2012b were conducted consecutively at East Malling Research with plots positioned 1500 m apart. An additional pre-established mapping population was phenotyped; the parents ‘Redgauntlet’ with moderate resistance to powdery mildew and ‘Hapil’ a susceptible cultivar were crossed to generate the RxH mapping population (168 individuals) which was phenotyped in 2012, 2013, 2014 and 2016 at East Malling (Sargent et al. 2012). Plants were maintained in a polytunnel and runners were pinned down into 9-cm pots containing compost and transferred into polythene covered raised beds with trickle fertigation (NPK, 22:4:22 at 25 kg ha−1). Raised beds were fumigated with chloropicrin to control soil-borne pests and diseases. Plants were trimmed in early July (or mid-April in Spain; 2013b) to remove old leaf material and expose young foliage; trimming ensures simultaneous disease development of new leaf material. Plantings were downwind of established strawberry plots, which provided a natural source of inoculum. Infection of mildew was allowed to establish within the field plots. Plants were arranged in a randomised block design with 3–6 replicate blocks per experiment, and each block contained a single replicate plant per genotype. Disease scores were recorded twice between late July and early September in UK field plots as dictated by disease symptom progression and twice during May in the Spanish plot. Plants were scored for mildew disease symptoms based on an existing scale (Simpson 1987), where scores denote: (1) a healthy plant with no visible disease symptoms, (2) slight leaf curling with no visible mycelium, (3) leaf curling and mottling, (4) severe leaf curling, redding and visible damage to lower leaf surface and (5) severe necrosis and some leaf death. A validation set of 75 cultivars and accessions were phenotyped in 2017 with ten replicate plants per accession.
Linkage map generation
DNA was extracted from new leaf material using the Qiagen DNAeasy plant mini extraction kit (Qiagen Ltd., Manchester, UK) according to the manufacturer’s instructions. Bi-parental populations were genotyped using the Affymetrix Istraw90 Axiom® array (i90k) containing approximately 90,000 potential genetic markers (Bassil et al. 2015). Cultivars and accessions were genotyped on the streamlined Axiom® IStraw35 384HT array (i35k), containing approximately 35,000 markers (Verma et al. 2017). The linkage maps were created using the Crosslink program (https://github.com/eastmallingresearch/crosslink) designed for octoploid linkage map development (Vickerstaff and Harrison 2017). The 28 linkage groups are denoted by chromosome number (1–7) and sub-genome (A–D) based on similarity to Fragaria vesca as described previously (Davik et al. 2015). Haplotype blocks lacking recombination in the progeny were identified for each mapping population and were used to identify neighbouring SNPs that may represent the same QTL and resistance allele in both mapping populations. In the ‘Redgauntlet’ × ‘Hapil’ linkage map, the average distance between markers is 0.75 cM; there are gaps > 20 cM on chromosome 1D, 4D and 6C. In the ‘Emily’ × ‘Fenella’ linkage map, the average distance between markers is 0.71 cM; there are gaps > 20 cM on chromosome 2C, 3B, 3D, 4D and 5C. The consensus map was generated using marker data from five mapping populations: ‘Redgauntlet’ × ‘Hapil’; EMR (Sargent et al. 2012), ‘Emily’ × ‘Fenella’; EMR, ‘Flamenco’ × ‘Chandler’; EMR, ‘Capitola’ × ‘CF1116’; INRA (Lerceteau-Köhler et al. 2003), ‘Camerosa’ × ‘Dover’; CRAG (Molina-Hidalgo et al. 2013). Python and R scripts used to generate the strawberry consensus map can be found at https://github.com/harrisonlab/ananassa_qtl. Marker positions were anchored to the F. vesca genome v2.0 (Tennessen et al. 2014), to allow the locations of QTL to be placed onto the physical map.
Statistical analysis
Phenotype calculation
The area under the disease progression curve (AUDPC) was calculated across the two scores for each phenotyping event using the R package ‘agricolae’ (de Felipe 2017) to predict scores for QTL analysis. AUDPC was calculated as follows (Shaner and Finney 1977):
where y is disease score, for score i, and X represents time in days and n is the number of scoring events. Relative AUDPC (rAUDPC) was calculated by dividing the AUDPC by the number of days after foliage trimming.
Spatial modelling
Spatial autocorrelation was assessed using Moran’s I test (R package ‘ape’; Paradis et al. 2004) based on the row- and column-field coordinates for each plant. Spatially corrected disease score estimates for each genotype were generated using REML and the AR1xAR1 model in Genstat (VSN International 2011) and used in downstream QTL analysis.
Combined analysis
Best linear unbiased estimates (BLUE) were generated for rAUDPC using a linear mixed effect model (R package ‘nlme’; Pinheiro et al. 2017) with genotype as a fixed effect and phenotyping event as a random effect; BLUEs provided overall disease scores per genotype, which were used for QTL analysis.
QTL identification
Disease resistance QTL were identified using Kruskal–Wallis analysis for each marker; the most significant marker was automatically selected for each linkage group and marker type before conducting stepwise AIC linear model selection in R. QTL effect size was calculated based on the output parameters from the linear regression between the observed phenotype of each genotype and predicted phenotype calculated based on identified QTL. The coefficients of determination (R2) associated with the linear regression provide a measure of the proportion of variance explained by the identified QTL. Epistatic interactions between significant QTL were identified through conducting a two-way analysis of variance (ANOVA).
Comparison of phenotyping events
Mixed effect models using the relative AUDPC values were used to assess the relative importance of genotype, environment and Genotype × Environment interactions on disease severity across phenotyping events. The models with and without each component, treating ‘Genotype’ as a fixed effect and ‘Year’ and ‘Genotype × Year’ as random effects, were compared using likelihood ratio test significance determined based on Chi-square values. Broad-sense heritability (H2) was calculated as H2 = VG/VP, where VG is the variance associated with genotype and VP is the total observed phenotypic variance, parameters calculated as part of an ANOVA. Normal residuals for AUDPC scores were confirmed using the Kolmogorov–Smirnov test. A two-way ANOVA on the relative AUDPC values allowed comparison of disease severity between phenotyping events to present comparative statistics in Fig. 1.
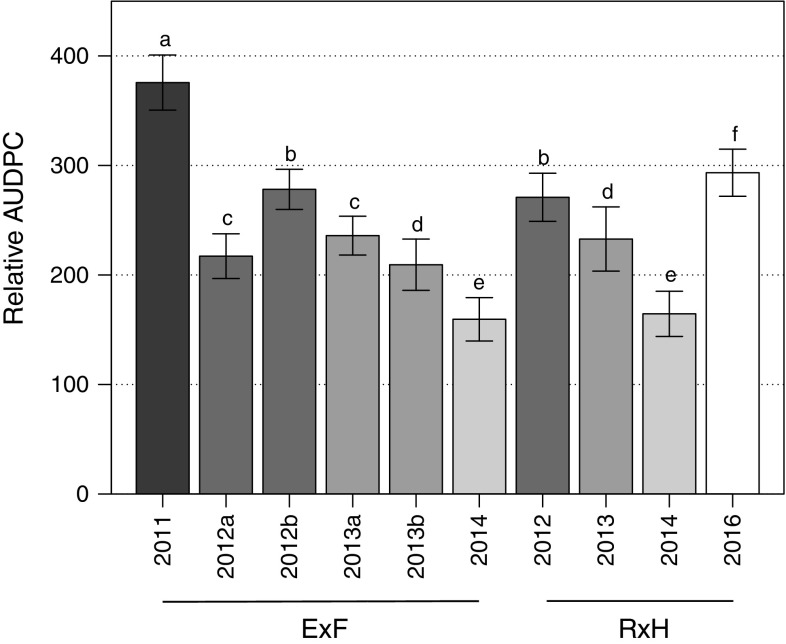
Fig. 1.
Average relative area under the disease progression curve (rAUDPC) across all genotypes for each phenotyping event. Error bars are standard errors
QTL validation
The QTL analysis is conducted using the i90k marker data in order to best represent the position of the resistance marker; however, the QTL analysis was repeated using the subset of i90k markers represented in the i35k chip and validation set, allowing the identification of substitute i35k markers associated with each QTL and comparison with an expanded validation panel of cultivars. Substitute i35k markers are on the same haplotype as the QTL identified in the i90k analysis in the mapping population; therefore, they are analogous but may have a weaker association with resistance due to their increased genetic distance from the ‘best’ marker in the mapping population. Restricted maximum likelihood (REML) was used to determine the strength of association between the substitute focal SNPs and the resistance allele in the wider germplasm using the R package ‘lme4’ (Bates et al. 2015) allowing the identification of markers in linkage disequilibrium (LD) with the QTL. A linear model between the observed and predicted phenotype was produced for QTL with strong marker–trait associations.
Association study
The subset of SNPs present on the Istraw90k chip, showing at least 10% minor allele frequency, was screened for association with mildew resistance (https://github.com/harrisonlab/popgen/blob/master/snp/gwas_quantitative_pipeline.md). This association analysis was conducted on 75 validation accessions using Plink (Purcell et al. 2007), and p values were corrected for population structure and adjusted using the Benjamini–Hochberg multiple test correction. Population structure was modelled by using pairwise identity by descent distance to allow multidimensional scaling, thus providing a quantitative indicator of genetic variation which was used as a covariate in downstream analysis.
Identification of candidate resistance genes
NB-LRR, TM–CC, RLP and RLK (S-type and general) were identified within the F. vesca genome (assembly v1.1) (Shulaev et al. 2011) by screening gene models for motifs following established pipelines (Li et al. 2016). Candidate susceptibility factors candidate MLO genes identified in Rosaceous crops by Pessina et al. (2014), and resistance genes were identified within 100 kbp of the significant QTL using BEDtools (Quinlan and Hall 2010) and tblastx (Karlin and Altschul 1993) against the NCBI database to determine any characterised function of homologous genes.
Results
Disease pressure varies between years and sites
The greatest proportion of variance in the relative AUDPC values was explained by the environment for both populations (‘Emily’ × ‘Fenella’: X2(1) = 1264.7; p < 0.001, ‘Redgauntlet’ × ‘Hapil’: X2(1) = 374.83; p < 0.001) followed by genotype (‘Emily’ × ‘Fenella’: X2(1) = 322.97; p < 0.001, ‘Redgauntlet’ × ‘Hapil’: X2(1) = 143.96; p < 0.001). The phenotyping events in 2014 showed low disease symptoms across both populations, suggesting either low disease pressure or low environmental conductivity (Fig. 1). A significant effect of Genotype × Environment (represented by phenotyping event) interaction was observed in both populations (‘Emily’ × ‘Fenella’: X2(1) = 170.66; p < 0.001, ‘Redgauntlet’ × ‘Hapil’: X2(1) = 29.11; p < 0.001). Across phenotyping events, broad-sense heritability varied between 24.1 and 59.0 for ‘Emily’ × ‘Fenella’ and 40.1–53.8 for ‘Redgauntlet’ × ‘Hapil’, revealing a moderate proportion of the variation in the data can be explained by the genetic variation and that there is a moderate to large environmental influence on disease symptom expression (Table 1). Nonetheless, the correlation analysis showed significant, positive correlations between disease scores for all the phenotyping events (Fig. 2).
Table 1.
Model parameters for the predictive linear model for each phenotyping event
| Mapping population | Year | R 2 | df | F value | p value | RSE | H 2 |
|---|---|---|---|---|---|---|---|
| E × F | 2011 | 0.23 | 3,146 | 14.42 | 2.8 × 10−8 | 5.28 | 73 |
| 2012a | 0.25 | 3,159 | 17.80 | 5.2 × 10−10 | 3.49 | 21 | |
| 2012b | 0.54 | 9,167 | 22.45 | 2.2 × 10−16 | 2.84 | 28 | |
| 2013a | 0.57 | 9,137 | 20.63 | 2.2 × 10−16 | 5.45 | 47 | |
| 2013b | 0.36 | 5,153 | 17.06 | 2.2 × 10−13 | 10.19 | 40 | |
| 2014 | 0.14 | 2,145 | 11.67 | 2.0 × 10−05 | 23.45 | 45 | |
| Blue | 0.46 | 6,172 | 24.72 | 2.2 × 10−16 | 29.62 | ||
| R × H | 2012 | 0.39 | 5,146 | 18.78 | 2.2 × 10−14 | 9.75 | 54 |
| 2013 | 0.25 | 3,155 | 17.01 | 1.3 × 10−09 | 8.17 | 29 | |
| 2014 | 0.25 | 2,156 | 25.28 | 2.5 × 10−10 | 25.28 | 56 | |
| 2016 | 0.16 | 1,107 | 20.50 | 1.6 × 10−5 | 7.02 | 35 | |
| Blue | 0.41 | 5,161 | 22.71 | 2.2 × 10−16 | 36.95 |
Predicted versus observed disease scores for each genotype within the population. R2 is the coefficient of determination; df are the degrees of freedom associated with the F statistic; the numerator is associated with model parameter number. H2 is broad-sense heritability associated with each phenotyping event
Fig. 2.
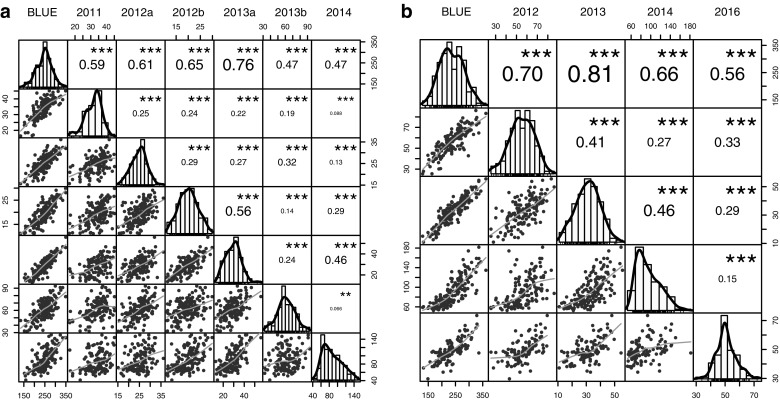
Pearson correlation matrix of powdery mildew area under the disease progression curve phenotype data for the strawberry mapping populations. a ‘Emily’ × ‘Fenella’ and b ‘Redgauntlet’ × ‘Hapil’. Numbers are R2 values
Stable and transient QTL are detected in the individual analyses
Five stable QTL (FaRPa2A, FaRPa5B, FaRPa6D1, FaRPa6D2, FaRPa7D1) were associated with powdery mildew resistance in more than one phenotyping event and the combined analysis. The focal SNP associated with powdery mildew resistance, representing the stable QTL FaRPa6D2, was consistently identified on linkage group 6D in ‘Redgauntlet’ across four phenotyping events and in the combined analysis. This allele is situated within 9 kbp of a putative resistance gene containing an RLK domain on chromosome six of the F. vesca genome. Highly significant QTL in ‘Emily’ were identified between 0.3 and 5.9 Mb on linkage group 1C ‘0’ haplotype in four individual phenotyping events and the combined analysis (Table 2 & Sup. Table 1). QTL on linkage group 1C represent some of the most significant markers associated with mildew resistance found in this study; however, the QTL position shifts depending upon the phenotyping event. Five focal SNPs were detected in two or more individual phenotyping events and multiple transient QTL were detected (Supp. Table 1).
Table 2.
Focal single nucleotide polymorphisms linked with each quantitative trait locus associated with strawberry powdery mildew disease resistance identified through the Kruskal–Wallis analysis using the best linear unbiased estimation calculated across all phenotyping events
| QTL name | Linkage group | Closest SNP | Position (Mb) | H | Sig | Parent | Percentage change | PRE | Closest R/S gene (kb) | Type of gene | Gene name | Number R genes 100 kb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FaRPa1C | 1C | Affx.88811437 | 3.2 | 24.4 | ****** | Emily | − 8.6 | 9.8 | 76 | RLK | mrna11302.1-v1.0-hybrid | 2 |
| FaRPa2A | 2A | Affx.88826254 | 20.3 | 18.0 | **** | Hapil | − 10.3 | 15.6 | Inside | TMCC | mrna10588.1-v1.0-hybrid | 1 |
| FaRPa2C | 2C | Affx.88879339 | 23.3 | 11.2 | *** | Emily | − 6.2 | 8.9 | 0.7a | TMCCa | maker-LG6-augustus-gene-134.221-mRNA-1a | 1 |
| FaRPa3A | 3A | Affx.88831545 | 1.5 | 10.0 | ** | Fenella | − 5.6 | 10.1 | Inside | RLK | maker-LG3-augustus-gene-0.106-mRNA-1 | 4 |
| FaRPa4B | 4B | Affx.88857340 | 28.5 | 13.0 | *** | Redgauntlet | − 6.6 | 6.5 | 22 | RLK | maker-LG4-snap-gene-259.168 | 2 |
| FaRPa5B | 5B | Affx.88862333 | 8.6 | 15.2 | **** | Fenella | − 6.5 | 11.4 | 2 | TMCC | mrna25962.1-v1.0-hybrid | 2 |
| FaRPa6D1 | 6D | Affx.88880958 | 14.7 | 12.0 | *** | Fenella | − 5.9 | 16.7 | 68 | RLP | augustus_masked-LG6-processed-gene-174.28-mRNA-1 | 2 |
| FaRPa6D2 | 6D | Affx.88904022 | 38.9 | 23.3 | ***** | Redgauntlet | − 11.2 | 7.2 | 9 | RLK | maker-LG6-augustus-gene-381.175 | 3 |
| FaRPa7C | 7C | Affx.88899370 | 18.7 | 8.4 | ** | Redgauntlet | 6.3 | 6.6 | 0.5 | RLK | mrna21020.1-v1.0-hybrid | 3 |
| FaRPa7D1 | 7D | Affx.88902178 | 20.9 | 18.9 | **** | Emily | − 6.9 | 8.9 | NA | NA | NA | 0 |
| FaRPa7D2 | 7D | Affx.88898584 | 17.0 | 12.2 | *** | Hapil | − 6.4 | 18.0 | 72 | NBS | augustus_masked-LG7-processed-gene-159.7-mRNA-1 | 2 |
| FaRPa6C | 6C | Affx.88882971 | 21.8 | 16.7 b | ***** | Validation | NA | NA | 44 | RLP | mrna15948.1-v1.0-hybrid | 2 |
Closest resistance gene reported within 100 kbp if applicable. H denotes the Kruskal–Wallis test statistic. Sig denotes the significance value associated with the marker: *p < 0.05 **p < 0.01; ***p < 0.001; ****p < 0.0001; *****p < 0.00001; ******p < 0.000001. Bold entries denote a focal single nucleotide polymorphisms linked with quantitative trait loci associated with strawberry powdery mildew disease resistance identified through the targeted marker association study
aMapped to LG6 not LG2
bt test statistic from plink analysis
In the combined analysis six QTL identified in the ‘Emily’ × ‘Fenella’ mapping population (Fig. 3) with a combined effect of 39.7% [proportional reduction of error (PRE) 66%], whereas the combined effect of the five QTL identified in the ‘Redgauntlet’ × ‘Hapil’ mapping population was 40.8% (PRE 54%; Fig. 4). Of the 12 QTL identified, 11 were associated with putative resistance genes in F. vesca, two of which fall inside a resistance gene (Table 2). Although none of the identified QTL fall within the assigned threshold of the putative MLO genes, FaRP2A is 133 kb away from the MLO-like protein gene 04.XM_004290718.2. The combined analysis pulled out seven QTL identified in at least one of the individual phenotyping events and four novel QTL. An epistatic interaction was found between the QTL FaRPa4B and FaRPa7D1 (F(1,151) = 6.65, p = 0.01) identified in the combined analysis of the ‘Redgauntlet’ × ‘Hapil’ population where individuals containing both QTL exhibit equivalent resistance to the those containing a single QTL. All other QTL are controlled by additive genetic components.
Fig. 3.
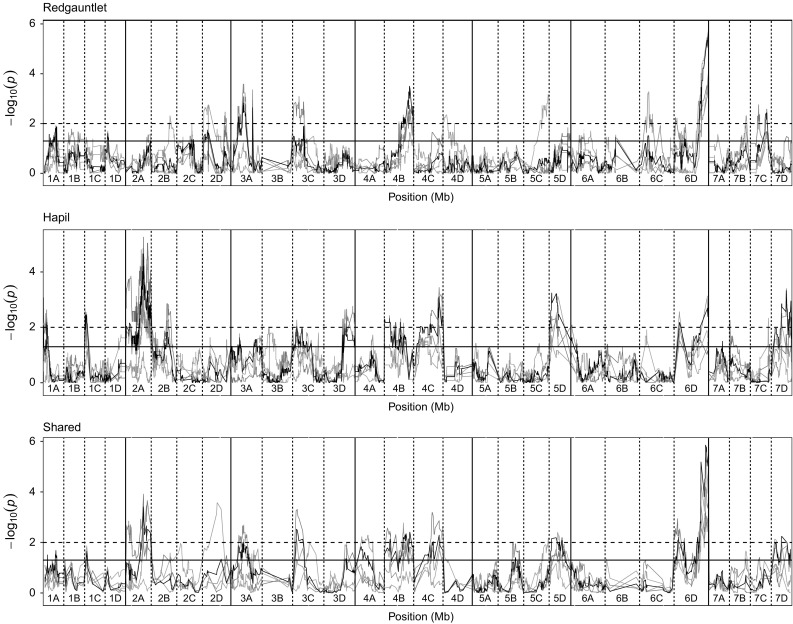
Kruskal–Wallis − log10 p values denoting the association of single nucleotide polymorphism with strawberry powdery mildew disease scores at each position in the octoploid strawberry genome in cM. Panels represent markers segregating in ‘Redgauntlet’, ‘Hapil’ and both parents. Labels 1A–7D denote the 28 linkage groups. Solid horizontal line is p = 0.05; dashed horizontal line is p = 0.01. Black line denotes combined analysis using the best linear unbiased estimates calculated across all phenotyping events. Colour denotes phenotyping event blue—2012, teal—2013, green—2014, pink—2016 (colour figure online)
Fig. 4.
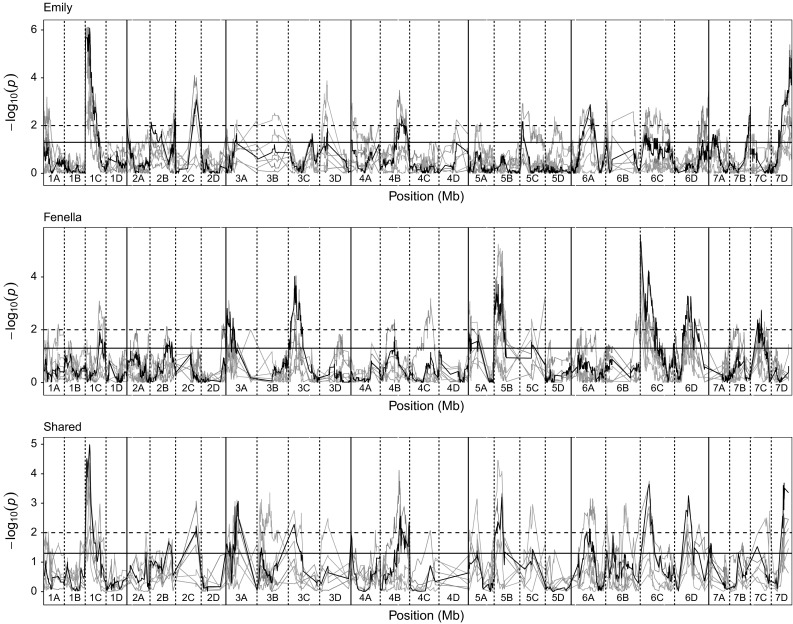
Kruskal–Wallis − log10 p values denoting the association of single nucleotide polymorphism with strawberry powdery mildew disease scores at each position in the octoploid strawberry genome in cM. Panels represent markers segregating in ‘Emily’, ‘Fenella’ and both parents. Labels 1A–7D denote the 28 linkage groups. Solid horizontal line is p = 0.05; dashed horizontal line is p = 0.01. Black line denotes combined analysis using the best linear unbiased estimates calculated across all phenotyping events. Colour denotes phenotyping event olive green—2011, light blue—2012a, green—2012b, red—2013a, blue—2013b (Spain), pink—2014 (colour figure online)
Detected QTL explain a large portion of the observed phenotypic variation
The coefficients of determination (R2) for the linear regression models of all phenotyping events show positive relationships between predicted and observed values, with between 16 and 57% of variation in observed scores explained by the identified QTL (Table 1).
Some QTL are detected in similar regions across the two populations
Focal ‘neighbouring’ SNPs on 7D from the ‘Redgauntlet’ × ‘Hapil’ and ‘Emily’ × ‘Fenella’ populations were identified to be 3.9 Mb away in the combined analysis (Fig. 5). The neighbouring focal markers on 7D are present on different parents in the ‘Redgauntlet’ × ‘Hapil’ population and are 15 cM apart; therefore, we cannot conclude whether the QTL identified in the two populations represent the same QTL.
Fig. 5.
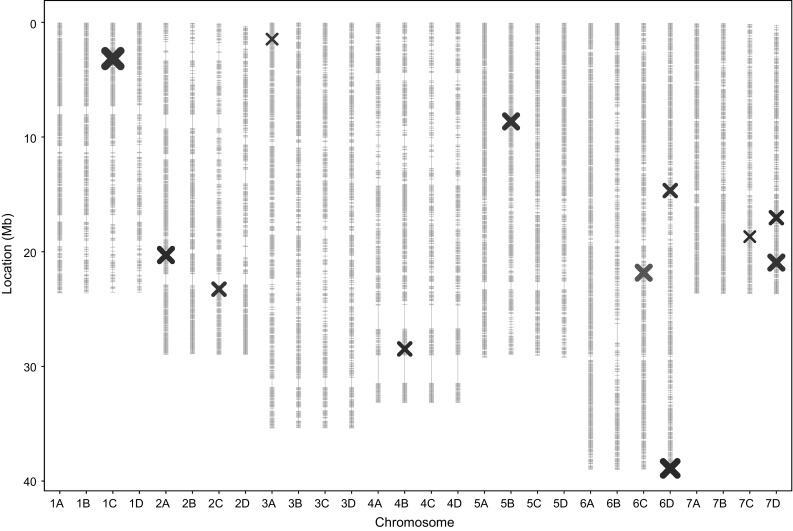
Linkage map displaying 35154 marker positions (grey) in Mb for 28 linkage groups of octoploid strawberry (1A–7D) marker positions scaled to the F. vesca genome. QTL locations from combined analysis ‘Emily’ × ‘Fenella’ (red) and ‘Redgauntlet’ × ‘Hapil’ (purple) and from the targeted marker association analysis (black) point size denote significance level of QTL (colour figure online)
QTL are poorly associated with phenotype in the wider germplasm
The combined transferable QTL analysis for the ‘Emily’ × ‘Fenella’ population produced two i35k substitute SNPs co-localising with focal SNPs identified in the i90k analysis, whereas the position of four focal SNPs had shifted (Sup. Fig 2). The combined transferable QTL analysis for the ‘Redgauntlet’ × ‘Hapil’ population produced four i35k focal SNPs co-localising with those identified in the i90k analysis, and one focal SNPs location had shifted. This analysis was associated with a slight loss in power to detect QTL positions but allowed QTL to be screened across the wider germplasm. One of the focal SNPs identified in the combined analysis on linkage group 7C maintained a weak association (X2(5) = 6.32; p = 0.067) with resistance across the wider germplasm. This QTL explained 29.2% of the variation in disease scores observed in the validation germplasm where two copies of the allele were present. The association analysis of the validation set identified multiple SNPs representing a single locus on linkage group 6C (Fig. 6; Table 2); however, this locus does not appear to contribute to the resistance of the mapping populations.
Fig. 6.
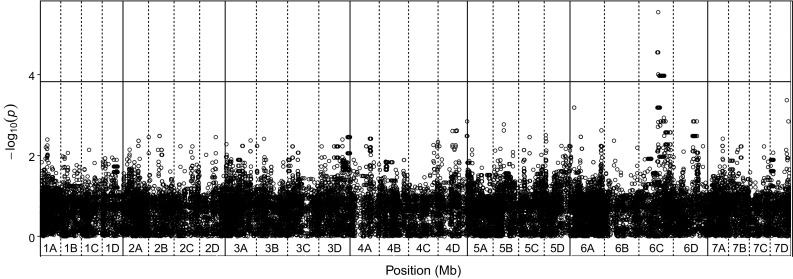
Unadjusted − log10 p values from plink denoting the association of single nucleotide polymorphism with strawberry powdery mildew disease scores at each position in the octoploid strawberry genome in cM, where known. Labels 1A–7D denote the 28 linkage groups. Solid horizontal line is p = 0.05 after Benjamini–Hochberg correction
Discussion
Comparison of disease scores across phenotyping events revealed the presence of a Genotype × Environment interaction in both populations. This was also observed in similar experiments from other groups (Kennedy et al. 2013). The variation in phenotypic scores between each experiment can be explained by differences in a combination of (1) the genetic diversity of the inoculum source, (2) inoculum load and (3) environmental conditions.
Each phenotyping experiment relied on natural inoculum from nearby plantings resulting in a different admixture of inoculum. There has been no report of race structure between P. aphanis and strawberry to date; furthermore, isolates from Italy and Israel were found to be homogenous after attempts to develop five discriminatory markers revealed monomorphic loci (Fiamingo et al. 2007). It was hypothesised that this may be attributed to low genetic variability in strawberry germplasm or low variation between mildew isolates (Xu et al. 2008b). However, there is some evidence to suggest heterogeneity between populations of P. aphanis, namely the evolution of fungicide resistance (Sombardier et al. 2010) and the resistance breaking in the cultivar Korona, likely due to the evolution of more virulent strains (Davik and Honne 2005). Reports of the production of ascospores in UK infections indicate the presence of a sexual cycle within powdery mildew (Xu et al. 2008a), and high recombination associated with this life cycle typically leads to greater genetic diversity than observed in asexual reproduction (Barrett et al. 2008). Qualitative resistance is associated with race-specific interactions, and typically the resistance is non-durable due to the R gene targeting a dispensable effector gene (Geiger and Heun 1989; Vleeshouwers et al. 2011). It was observed that resistance to P. aphanis within new strawberry selections is not durable over time and across varying environmental conditions; however, it cannot be determined whether this is due to unstable resistance or variable mildew strains (McNicol and Gooding 1979; Nelson et al. 1995; Xu et al. 2008b). These examples indicate the requirement for constant breeding and selection for powdery mildew resistance in strawberry.
It has been suggested that different genes confer resistance to mildew depending on the inoculum level (Nelson et al. 1995, 1996; Kennedy et al. 2013). Due to the natural inoculation method, phenotyping events varied in inoculum load and such variation may create differential induction of systemic resistance. Future mildew infection experiments could aim to quantify field inoculum levels to qualify resistance genes as effective under low or high inoculum levels. The infection levels of neighbouring plants will influence the inoculum load experienced by a given plant and therefore the resistance status (Hughes et al. 1997). The trimming of older leaves from plants has allowed greater uniformity in disease symptom progression to mitigate the potential for uneven inoculum load. However, significant spatial autocorrelation was observed and plant disease scores were corrected accordingly.
Environmental conditions affect the sporulation, germination and establishment of P. aphanis conidia. Optimum conditions for germination occur between 75 and 98% RH and between 15 and 25 °C with disease symptoms observed 4 days after germination (Amsalem et al. 2006). Expression of quantitative disease resistance is influenced by soil, weather and age of plant material (Geiger and Heun 1989). The plants in each phenotyping event will be exposed to different environmental conditions. However, the two phenotyping events 2012a and 2012b were conducted within 500 m and therefore have experienced a similar macro-environment and inoculum admixture. The coefficient of determination of 29% between 2012a and 2012b phenotyping events indicates a moderate correlation of disease score.
A strong correlation was observed between strawberry cultivar powdery mildew resistance assessed in the field and polytunnel environments (Gooding et al. 1981; Nelson et al. 1996; Kennedy et al. 2013). Therefore, our powdery mildew field assessments should reflect resistance levels exhibited in a polytunnel environment and support transferability of resistance alleles from the field into the polytunnel. Furthermore, plant nutrient status has been found to impact mildew-resistant status. Indeed, low calcium levels were associated with weakened mildew resistance response in the cultivar ‘Aroma’ (Palmer 2007). However, fertigation was applied to all plots mitigating the potential for plant nutrient status to impact mildew disease scores.
The QTL analysis was performed individually for each phenotyping event, allowing the identification of both stable and transient QTL. A different suite of QTL from ‘Emily’ × ‘Fenella’ was identified as significant for each of the six phenotyping events, indicating that the resistance is indeed complex and quantitative. However, four QTL have support across multiple years of phenotyping. Therefore, relatively few stable QTL are observed alongside multiple transient QTL. Stable and transient QTL were also found to control apple powdery mildew resistance (Calenge and Durel 2006). Stable QTL represent alleles involved in disease resistance on the majority of infection events; however, transient QTL may represent genes that have an environment-specific interaction and as such are important in only some infection events. The large number of transient QTL may be attributed to the variation in inoculum source, inoculum load and environmental conditions between treatments. We found multiple QTL of small to moderate effect control disease resistance in both populations. Likewise, previous studies have found multiple small effect QTL which contribute to powdery mildew resistance in strawberry indicating quantitative resistance with both additive and non-additive genetic components (Simpson 1987; Nelson et al. 1995; Davik and Honne 2005).
Previous studies have shown ‘Hapil’ has a low estimated breeding value for mildew resistance of 0.036 (± 0.095), showing almost no genetic component for mildew susceptibility status in this cultivar (Davik and Honne 2005). Here, we show several QTL associated with resistance can be identified in the ‘Hapil’ cultivar, indicating that there are some powdery mildew resistance genes in this cultivar that could be exploited by breeders. Heritability observed in this current study is lower than the field-based disease severity in studies conducted by Nelson et al. (1995) of 0.7, which indicates a strong genetic component. It has also been found that higher heritability values are associated with high infection levels due to a greater uniform inoculum distribution (Nelson et al. 1995). Due to the natural inoculation process, there is the potential for a patchy inoculum dispersal across the field site, and this may explain relatively low heritability scores.
Genotyping and field phenotyping of the validation accessions revealed that one of the resistance QTL was associated with resistance within the wider strawberry germplasm, indicating a strong candidate marker for further study. However, the low transferability of markers highlights the likelihood for multiple sources of powdery mildew resistance across strawberry germplasm and the need for an enhanced panel of genetic markers that fully represent the diversity present in strawberry germplasm.
When validating QTL identified in a bi-parental cross over the wider germplasm, a reduction in the number of informative loci is anticipated due to the lack of LD between the markers ‘tagging’ resistance in a bi-parental population and the QTL. Based upon the design of the i90k and subsequent arrays, it is likely that these only capture a tiny fraction of the genetic variation, based on the limited discovery panel that was used during the array design (Bassil et al. 2015). The focus on the selection of common SNPs for the i35k array also means that low-frequency markers present on the i90k are also absent (Verma et al. 2017). Furthermore, if QTL are at low frequencies in the wider population, it is unlikely that the underpowered preliminary association study that we have carried out will have sufficient power to detect QTL.
Nonetheless, here we observe one conserved resistance QTL across the wider strawberry germplasm and highlight the potential for some limited transferability of markers into resistance breeding. Future work will seek to identify the candidate resistance genes associated with the QTL on linkage group 1C and 6D and screen for presence of more candidate resistance genes across the wider germplasm. Work will seek to identify the mechanism of resistance in the cultivars ‘Emily’ and ‘Redgauntlet’. Such work has been conducted on the powdery mildew-resistant cultivar ‘Aroma’, where poor colony establishment was associated with the identification of a putative antimicrobial protein (Palmer 2007). Additionally, lower conidial attachment was associated with high cutin acid in ‘Aroma’ leaf cuticles; indeed, high cutin acid has been extracted from many resistant strawberry cultivars (Peries 1962; Jhooty and McKeen 1965). Further to this, relatively high powdery mildew resistance was observed in Florida cultivars from wild accessions of Fragaria virginiana after successful introgression into strawberry breeding germplasm, highlighting the potential of natural reserves of resistance to be used in crop breeding programmes (Kennedy et al. 2013).
The magnitude of disease symptom variation shows great potential to enhance mildew resistance. The most resistant accession was 3.2 times more resistant than the most susceptible within the validation accessions. We conclude that multiple QTL of small effect control disease resistance and that principally a different suite of alleles control resistance in the two studied populations, with a limited overlap. The small effect size of loci promotes a genomic selection breeding approach for powdery mildew resistance in strawberry, as this may be most effective at capturing the wide range of small effect QTL that are likely to be present in a breeding programme. Any training population would need to be phenotyped in multiple environments and years in order to capture the diverse expression of QTL and fully maximise the power of a GS approach. Furthermore, a more detailed study of the pathogen’s population structure and host interactions is needed to quantify the contribution of pathogen diversity to transient QTL.
Ultimately, the production of mildew-resistant strawberry cultivars will reduce grower reliance on chemical fungicide for control of powdery mildew; such control options are particularly important with respect to reducing consumer concerns over pesticide residues and also where deregulation of existing fungicide actives is reducing disease management options.
Author contribution statement
RJH, DWS and DJS conceived, designed and analysed experiments. AJP propagated plant material. KJM, NH, KP, ML, AK, HMC and JH recorded pathogenicity data in field experiments. RJV and AK analysed SNP data and made linkage map. HMC was involved in QTL mapping and statistical analysis. FW, ML and LA extracted gDNA for SNP chip analysis. MKS created R gene database and pipeline used for plink analysis. HMC and RJH wrote the manuscript. It was not possible to contact ML, KJM and LA for approval.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Linkage map displaying 35154 marker positions (grey) in Mb for 28 linkage groups of octoploid strawberry (1A–7D) marker positions scaled to F. vesca genome. QTL locations from each phenotyping event represented (PDF 207 kb)
Linkage map displaying marker positions (grey) in Mb for 28 linkage groups of octoploid strawberry (1A–7D) marker positions scaled to F. vesca genome. Markers overlapping between the validation set and ‘Emily’ x ‘Fenella’ and ‘Redgauntlet’ x ‘Hapil’ populations are red and blue ‘-’, respectively. QTL locations from combined analysis ‘Emily’ x ‘Fenella’ (red) and ‘Redgauntlet’ x ‘Hapil’ (purple) (PDF 263 kb)
Acknowledgements
The authors acknowledge funding from the Biotechnology and Biological Sciences Research Council (BBSRC) BB/K017071/1, BB/N006682/1 and Innovate UK Project 100875. The authors acknowledge Philip Brain, Eric Van de Weg and Xiangming Xu for statistical advice. The authors also acknowledge INRA and CRAG for contributing their mapping population data to the strawberry consensus linkage map.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Amano K. Host range and geographical distribution of the powdery mildew fungi. Tokyo: Japan Scientific Societies Press; 1986. [Google Scholar]
- Amsalem L, Freeman S, Rav-David D, et al. Effect of climatic factors on powdery mildew caused by Sphaerotheca macularis f. sp. Fragariae on strawberry. Eur J Plant Pathol. 2006;114:283–292. doi: 10.1007/s10658-005-5804-6. [DOI] [Google Scholar]
- Asalf B, Gadoury DM, Tronsmo AM, et al. Ontogenic resistance of leaves and fruit, and how leaf folding influences the distribution of powdery mildew on strawberry plants colonized by Podosphaera aphanis. Phytopathology. 2014;104:954–963. doi: 10.1094/PHYTO-12-13-0345-R. [DOI] [PubMed] [Google Scholar]
- Barrett LG, Thrall PH, Burdon JJ, Linde CC. Life history determines genetic structure and evolutionary potential of host-parasite interactions. Trends Ecol Evol (Amst) 2008;23:678–685. doi: 10.1016/j.tree.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil NV, Davis TM, Zhang H, et al. Development and preliminary evaluation of a 90 K Axiom® SNP array for the allo-octoploid cultivated strawberry Fragaria × ananassa. BMC Genom. 2015;16:1310. doi: 10.1186/s12864-015-1310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Braun U. A monograph of the Erysiphales (powdery mildews) Stuttgart: Schweizerbart Science Publishers; 1987. [Google Scholar]
- Calenge F, Durel CE. Both stable and unstable QTLs for resistance to powdery mildew are detected in apple after four years of field assessments. Mol Breeding. 2006;17:329–339. doi: 10.1007/s11032-006-9004-7. [DOI] [Google Scholar]
- Calleja EJ (2011) The potential impacts of climate change on diseases affecting strawberries and the UK strawberry industry. Doctoral dissertation
- Colla P, Gilardi G, Gullino ML. A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica. 2012;40:515–523. doi: 10.1007/s12600-012-0252-2. [DOI] [Google Scholar]
- Davik J, Honne BI. Genetic variance and breeding values for resistance to a wind-borne disease [Sphaerotheca macularis (Wallr. ex Fr.)] in strawberry (Fragaria × ananassa Duch.) estimated by exploring mixed and spatial models and pedigree information. Theor Appl Genet. 2005;111:256–264. doi: 10.1007/s00122-005-2019-3. [DOI] [PubMed] [Google Scholar]
- Davik J, Sargent DJ, Brurberg MB, Lien S, Kent M, Alsheikh M. A ddRAD based linkage map of the cultivated strawberry, Fragaria × ananassa. PLoS ONE. 2015;10(9):e0137746. doi: 10.1371/journal.pone.0137746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe M (2017) agricolae: Statistical Procedures for Agricultural Research. CRAN, R package
- Fiamingo F, Amsalem L, Maymon M, et al. Sensitivity of two Podosphaera aphanis populations to disease control agents. J. Plant Pathol. 2007;89:85–96. [Google Scholar]
- Geiger HH, Heun M. Genetics of quantitative resistance to fungal diseases. Annu Rev Phytopathol. 1989;27:317–341. doi: 10.1146/annurev.py.27.090189.001533. [DOI] [Google Scholar]
- Gooding HJ, McNicol RJ, et al. Methods of screening strawberries for resistance to Sphaerotheca maculons (Wall Ex Frier) and Phytophthora cactorum (Leb. and Cohn) J Hortic Sci. 1981;56:239–245. doi: 10.1080/00221589.1981.11514995. [DOI] [Google Scholar]
- Harvey N, Xu X. Powdery mildew on raspberry is genetically different from strawberry powdery mildew. J Plant Pathol. 2010;92:775–779. [Google Scholar]
- Hughes G, McRoberts N, Madden LV, et al. Validating mathematical models of plant-disease progress in space and time. Math Med Biol. 1997;14:85–112. doi: 10.1093/imammb/14.2.85. [DOI] [Google Scholar]
- Jhooty JS, McKeen WE. The influence of host leaves on germination of the asexual spores of Sphaerotheca macularis (Wallr. ex. Fr.) Cooke. Can J Microbiol. 1965;11:539–545. doi: 10.1139/m65-071. [DOI] [PubMed] [Google Scholar]
- Karlin S, Altschul SF. Applications and statistics for multiple high-scoring segments in molecular sequences. Proc Natl Acad Sci USA. 1993;90:5873–5877. doi: 10.1073/pnas.90.12.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Hasing TN, Peres NA, et al. Evaluation of strawberry species and cultivars for powdery mildew resistance in open-field and high tunnel production systems. HortScience. 2013;48:1125–1129. [Google Scholar]
- Lainsbury MA. The pesticide guide. 29. Wallingford: CABI Publishing; 2016. [Google Scholar]
- Lerceteau-Köhler E, Guerin G, Laigret F, et al. Characterization of mixed disomic and polysomic inheritance in the octoploid strawberry (Fragaria × ananassa) using AFLP mapping. Theor Appl Genet. 2003;107(4):619–628. doi: 10.1007/s00122-003-1300-6. [DOI] [PubMed] [Google Scholar]
- Li P, Quan X, Jia G, et al. RGAugury: a pipeline for genome-wide prediction of resistance gene analogs (RGAs) in plants. BMC Genom. 2016;17:852. doi: 10.1186/s12864-016-3197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol RJ, Gooding HJ. Assessment of strawberry clones and seedlings for resistance to Sphaerotheca macularis (Wall ex Frier) Horticultural Research. 1979;19:35–41. [Google Scholar]
- Molina-Hidalgo FJ, Franco AR, Villatoro C, et al. The strawberry (Fragaria × ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell-wall middle lamellae. J Exp Bot. 2013;64(6):1471–1483. doi: 10.1093/jxb/ers386. [DOI] [PubMed] [Google Scholar]
- Mori Y, Sato Y, Takamatsu S. Evolutionary analysis of the powdery mildew fungi using nucleotide sequences of the nuclear ribosomal DNA. Mycologia. 2000;91:74–93. doi: 10.2307/3761452. [DOI] [Google Scholar]
- Nelson MD, Gubler WD, Shaw DV. Inheritance of powdery mildew resistance in greenhouse-grown versus field-grown California strawberry progenies. Phytopathology. 1995;85:421–424. doi: 10.1094/Phyto-85-421. [DOI] [Google Scholar]
- Nelson MD, Gubler WD, Shaw DV. Relative resistance of 47 strawberry cultivars to powdery mildew in California Greenhouse and field environments. Plant Dis. 1996;80:326–328. doi: 10.1094/PD-80-0326. [DOI] [Google Scholar]
- Palmer S (2007) Strawberry powdery mildew: epidemiology and the effect of host nutrition on disease. Doctoral dissertation, The University of Adelaide
- Paradis E, Claude J, Strimmer K. APE: analysis of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Paulus AO. Fungal diseases of strawberry. HortScience. 1990;25:885–889. [Google Scholar]
- Peries OS. Studies on strawberry mildew, caused by Sphaerotheca macularis (Wallr. ex Fries) Jaczewski. Annals of Applied Biology. 1962;50:211–224. doi: 10.1111/j.1744-7348.1962.tb06004.x. [DOI] [Google Scholar]
- Pessina S, Pavan S, Catalano D, et al. Characterization of the MLO gene family in Rosaceae and gene expression analysis in Malus domestica. BMC Genom. 2014;15:618. doi: 10.1186/1471-2164-15-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, et al (2017) nlme: linear and nonlinear mixed effects models. CRAN, R package
- Purcell S, Neale B, Todd-Brown K, et al. PLINK a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent DJ, Passey T, Surbanovski N, Lopez Girona E, Kuchta P, Davik J, Harrison R, Passey A, Whitehouse AB, Simpson DW. A microsatellite linkage map for the cultivated strawberry (Fragaria × ananassa) suggests extensive regions of homozygosity in the genome that may have resulted from breeding and selection. Theor Appl Genet. 2012;124(7):1229–1240. doi: 10.1007/s00122-011-1782-6. [DOI] [PubMed] [Google Scholar]
- Shaner G, Finney RE. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology. 1977;67(8):1051–1056. doi: 10.1094/Phyto-67-1051. [DOI] [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, et al. The genome of woodland strawberry (Fragaria vesca) Nat Genet. 2011;43:109–116. doi: 10.1038/ng.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DW. The inheritance of mildew resistance in everbearing and day-neutral strawberry seedlings. J Hortic Sci. 1987;62:329–334. doi: 10.1080/14620316.1987.11515788. [DOI] [Google Scholar]
- Sombardier A, Dufour M-C, Blancard D, Corio-Costet M-F. Sensitivity of Podosphaera aphanis isolates to DMI fungicides: distribution and reduced cross-sensitivity. Pest Manag Sci. 2010;66:35–43. doi: 10.1002/ps.1827. [DOI] [PubMed] [Google Scholar]
- Tennessen JA, Govindarajulu R, Ashman TL, Liston A. Evolutionary origins and dynamics of octoploid strawberry subgenomes revealed by dense targeted capture linkage maps. Genome Biol Evol. 2014;6(12):3295–3313. doi: 10.1093/gbe/evu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Bassil NV, Van De Weg E, et al. Development and evaluation of the Axiom® IStraw35 384HT array for the allo-octoploid cultivated strawberry Fragaria × ananassa. Acta Hortic. 2017;1156:75–82. doi: 10.17660/ActaHortic.2017.1156.10. [DOI] [Google Scholar]
- Vickerstaff RJ, Harrison RJ (2017) Crosslink: a fast, scriptable genetic mapper for outcrossing species. BioRxiv. 10.1101/135277
- Vleeshouwers VGAA, Raffaele S, Vossen JH, et al. Understanding and exploiting late blight resistance in the age of effectors. Annu Rev Phytopathol. 2011;49:507–531. doi: 10.1146/annurev-phyto-072910-095326. [DOI] [PubMed] [Google Scholar]
- VSN International (2011) GenStat for Windows 14th Edition. VSN International, Hemel Hempstead, UK. www.GenStat.co.uk
- Whitaker VM, Osorio LF, Hasing T. Estimation of genetic parameters for 12 fruit and vegetative traits in the University of Florida strawberry breeding population. J Am Soc Hortic Sci. 2012;137:316–324. [Google Scholar]
- Xiao CL, Chandler CK, Price JF, et al. Comparison of epidemics of Botrytis fruit rot and powdery mildew of strawberry in large plastic tunnel and field production systems. Plant Dis. 2001;85:901–909. doi: 10.1094/PDIS.2001.85.8.901. [DOI] [PubMed] [Google Scholar]
- Xu X, Robinson J, Berrie A. Potential role of cleistothecia in strawberry powdery mildew. IOBC/WPRS. 2008;39:211–215. [Google Scholar]
- Xu X, Robinson J, Simpson D. Interactions between isolates of powdery mildew (Podosphaera aphanis) and cultivars of strawberry, Fragaria × ananassa. IOBC Bulletin. 2008;39:203–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage map displaying 35154 marker positions (grey) in Mb for 28 linkage groups of octoploid strawberry (1A–7D) marker positions scaled to F. vesca genome. QTL locations from each phenotyping event represented (PDF 207 kb)
Linkage map displaying marker positions (grey) in Mb for 28 linkage groups of octoploid strawberry (1A–7D) marker positions scaled to F. vesca genome. Markers overlapping between the validation set and ‘Emily’ x ‘Fenella’ and ‘Redgauntlet’ x ‘Hapil’ populations are red and blue ‘-’, respectively. QTL locations from combined analysis ‘Emily’ x ‘Fenella’ (red) and ‘Redgauntlet’ x ‘Hapil’ (purple) (PDF 263 kb)